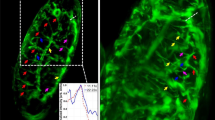
Abstract
A major challenge for fluorescence imaging of living mammalian cells is maintaining viability following prolonged exposure to excitation illumination. We have monitored the dynamics of mitochondrial distribution in hamster embryos at frequent intervals over 24 h using two-photon microscopy (1,047 nm) while maintaining blastocyst, and even fetal, developmental competence. In contrast, confocal imaging for only 8 h inhibits development, even without fluorophore excitation. Photo-induced production of H2O2 may account, in part, for this inhibition. Thus, two-photon microscopy, but not confocal microscopy, has permitted long-term fluorescence observations of the dynamics of three-dimensional cytoarchitecture in highly photosensitive specimens such as mammalian embryos.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Terasaki, M. & Dailey, M.E. in Handbook of biological confocal microscopy (ed. Pawley, J.B.) 327–346 (Plenum, New York; 1995).
Hillman, N. & Tasca, R. Ultrastructural and autoradiographic studies of mouse cleavage stages. Am. J. Anat. 126, 151–174 (1983).
Batten, B.E., Albertini, D.F. & Ducibella, T. Patterns of organelle distribution in mouse embryos during preimplantation development. Am. J. Anat. 178, 204–213 (1987).
Holy, J., Simerly, C., Paddock, S. & Schatten, G. Three-dimensional imaging of fertilization and early development. J. Electron Microsc. Technol. 17, 384–400 (1991).
Capco, D.G., Gallicano, G.I., McGaughey, R.W., Downing, K.H., & Larabell, C.A. Cytoskeletal sheets of mammailian eggs and embryos: a lattice-like network of intermediate filaments. Cell Motil. Cytoskeleton 24, 85–99 (1993).
Barnett, D., Kimura, J. & Bavister, B. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev. Dyn. 205, 64–72 (1996).
Svoboda, K., Denk, W., Kleinfeld, D. & Tank, D.W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385, 161–165 (1997).
Takada, T. et al. Selective production of transgenic mice using green fluorescent protein as a marker. Nat. Biotechnol. 15, 458–461 (1997).
Chalfie, M., Tu, Y., Euskirchen, G., Ward, W.W. & Prasher, D.C. Green fluorescent protein as a marker for gene expression. Science 263, 802–805 (1994).
Bavister, B. Culture of preimplantation embryos: facts and artifacts. Hum. Reprod. Update 1, 91–148 (1995).
Daniel, J.C. Cleavage of mammalian ova inhibited by visible light. Nature 201, 316–317 (1964).
Hirao, Y. & Yanagimachi, R. Detrimental effect of visible light on meiosis of mammalian eggs in vitro. J. Exp. Zool. 206, 365–369 (1978).
Hegele-Hartung, C., Schumacher, A. & Fischer, B. Effects of visible light and room temperature on the ultrastructure of preimplantation rabbit embryos: a time course study. Anat. Embryol. (Berl). 183, 559–571 (1991).
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Xu, C., Zipfel, W., Shear, J.B., Williams, R.M. & Webb, W.W. Multiphoton fluorescence excitation - new spectral windows for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA 93, 10763–10768 (1996).
Wokosin, D. et al. All-solid-state ultrafast lasers facilitate multiphoton excitation fluorescence imaging. Institute of Electrical and Electronics Engineering Journal of Selected Topics in Quantum Electronics. 2, 1051–1065 (1996).
Konig, K., So, P.T.C., Mantulin, W. & Gratton, E. Cellular response to near-infrared femtosecond laser pulses in two-photon microscopes. Optics Letters 22, 135–136 (1997).
Williams, R.M., Piston, D.W. & Webb, W.W. Two-photon molecular excitation provides intrinsic 3-dimensional resolution for laser-based microscopy and microphotochemistry. Faseb J. 8, 804–813 (1994).
Konig, K., Simon, U. & Halbhuber, K.J. 3D resolved two photon fluorescence microscopy of living cells using a modified confocal laser scanning microscope. Cell. Mol. Biol. 42, 1181–1194 (1996).
Barnett, D.K., Clayton, M.K., Kimura, J. & Bavister, B.D. Glucose and phosphate toxicity in hamster preimplantation embryos involves disruption of cellular organization, including distribution of active mitochondria. Mol. Reprod. Dev. 48, 227–237 (1997).
Wang, R.J. & Nixon, B.R. Identification of hydrogen peroxide as a photoproduct toxic to human cells in tissue-culture medium irradiated with "daylight" fluorescent light. In Vitro 14, 715–722 (1978).
Konig, K.K. et al. Cell damage by UVA radiation of a mercury microscopy lamp probed by autofluroescence modifications, cloning assay, and comet assay. J. Biomed. Optics 1, 217–222 (1996).
Hockberger, P.E. et al. Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc. Natl. Acad. Sci. USA 96, 6255–6260 (1999).
Nasr-Esfahani, M.H., Aitken, J.R. & Johnson, M.H. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development 109, 501–507 (1990).
Nasr-Esfahani, M.M. & Johnson, M.H. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development 113, 551–560 (1991).
Nakayama, T., Noda, Y., Goto, Y. & Mori, T. Effects of visible light and other environmental faxtors on the production of oxygen radicals by hamster embryos. Theriogeneology. 41, 499–510 (1994).
Rothe, G. & Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescein. J. Leukoc. Biol. 47, 440–448 (1990).
LeBel, C.P., Ischiropoulos, H. & Bondy, S.C. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 5, 227–231 (1992).
Zhu, H., Bannenberg, G.L., Moldeus, P. & Shertzer, H.G. Oxidation pathways for the intracellular probe 2',7'-dichlorofluorescein. Arch. Toxicol. 68, 582–587 (1994).
Haugland, R.P. Detecting enzymatic activity in cells using fluorogenic substrates. Biotechnics and Histochemistry. 70, 243–251 (1995).
Hockberger, P.E. et al. in Optical diagnostics of living cells and biofluids, SPIE International Society for Optical Engineering, (eds Asakura, T., Farkas, D.L., Lief, R.C., Priezzhev, A.V. & Tromberg, B.J.) 129–140, Bellingham, WA, (1996).
Aubin, J.E. Autofluorescence of viable cultured mammalian cells. J. Histochem. Cytochem. 27, 36–43 (1979).
Cathcart, R., Schwiers, E. & Ames, B.N. Detection of picomole levels of lipid hydroperoxides using a dichlorofluorescein fluorescent assay. Methods Enzymol. 105, 352–358 (1984).
Mohler, W. & Squirrell, J.M. in Imaging nerurons: a laboratory manual (eds Yuste, R., Lanni, K. & Konnerth, A.) (Cold Spring Harbor Press, Cold Spring Harbor, NY, in the press).
Bavister, B.D. A minichamber device for maintaining a constant carbon dioxide in air atmosphere during prolonged culture of cells on the stage of an inverted microscope. In Vitro Cellular and Devolpment Biology. 24, 759–763 (1988).
McKiernan, S. & Bavister, B. Pantothenate stimulates blastocyst formation in cultured one-cell hamster embryos. Theriogeneolgy. 49, 209 (1998).
Wokosin, D.L. & White, J.G. in Three-dimensional microscopy: image acquisition and processing, SPIE International Socity for Optical Engineering (eds Cogswell, C.J., Conchello, J.-A. & Wilson, T.) 24–29, Bellingham, WA; 1997).
Ludwig, T.E., Lane, M. & Bavister, B.D. Increased fetal development after transfer of hamster embryos cultured with glucose. Biol. Reprod. 58, 167 (1998).
Acknowledgements
The authors would like to thank Tenneille Ludwig for performing the embryo transfers, Kevin Eliceiri for technical assistance, Dr. Philip Hockberger for assistance with the peroxide study, and Drs. Jay Baltz, Victoria Centonze-Frohlich, Philip Hockberger, Keith Latham, Gary Lyons, Randall Prather, and Mark Westhusin for their comments on the manuscript. This work was supported by the NICHD National Cooperative Program on Non-Human In Vitro Preimplantation Embryo Development through grant HD22023 to BDB and the NIH grant RR00570 to the I.M.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Squirrell, J., Wokosin, D., White, J. et al. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat Biotechnol 17, 763–767 (1999). https://doi.org/10.1038/11698
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/11698
This article is cited by
-
Time-lapse observation of mouse preimplantation embryos using a simple closed glass capillary method
Scientific Reports (2023)
-
Culture conditions in the IVF laboratory: state of the ART and possible new directions
Journal of Assisted Reproduction and Genetics (2023)
-
Image improvement of temporal focusing multiphoton microscopy via superior spatial modulation excitation and Hilbert–Huang transform decomposition
Scientific Reports (2022)
-
Phototoxicity of BODIPY in long-term imaging can be reduced by intramolecular motion
Photochemical & Photobiological Sciences (2022)
-
The effect of discrete wavelengths of visible light on the developing murine embryo
Journal of Assisted Reproduction and Genetics (2022)