Abstract
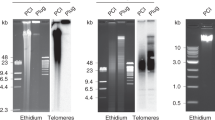
To measure the average length of telomere repeats at chromosome ends in individual cells we developed a flow cytometry method using fluorescence in situ hybridization (flow FISH) with labeled peptide nucleic acid (PNA) probes. Results of flow FISH measurements correlated with results of conventional telomere length measurements by Southern blot analysis (R=0.9). Consistent differences in telomere length in CD8+ T-cell subsets were identified. Naive and memory CD4+ T lymphocytes in normal adults differed by around 2.5 kb in telomere length, in agreement with known replicative shortening of telomeres in lymphocytes in vivo. T-cell clones grown in vitro showed stabilization of telomere length after an initial decline and rare clones capable of growing beyond 100 population doublings showed variable telomere length. These results show that flow FISH can be used to measure specific nucleotide repeat sequences in single cells and indicate that the very large replicative potential of lymphocytes is only indirectly related to telomere length.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blackburn, E.H. and Greider, C.W. (eds.). 1995. Telomeres. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Harley, C.B., Futcher, A.B. and Greider, C.W. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460.
Allsopp, R.C., Vaziri, H., Patterson, C., Goldstein, S., Younglai, E.V., Futcher, A.B. et al. 1992. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 89: 10114–10118.
Hastie, N.D., Dempster, M., Dunlop, M.G., Thompson, A.M., Green, D.K. and Allshire, R.C. 1990. Telomere reduction in human colorectal carcinoma and with ageing. Nature 346: 866–868.
Vaziri, H., Schachter, F., Uchida, I., Wei, L., Zhu, X., Effros, R. et al. 1993. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am. J. Hum. Genet. 52: 661–667.
Greider, C.W. and Blackburn, E.H. 1985. Identification of a specific telomere terminal transferase activity in Tetrhymena extracts. Cell 43: 405–413.
Lingner, J., Hughes, T.R., Shevchenko, A., Mann, M., Lundblad, V. and Cech, T.R. 1997. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276: 561–567.
Blasco, M.A., Lee, H.-W., Hande, M.P., Samper, E., Lansdorp, P.M., DePinho, R.A. et al. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25–34.
Autexier, C. and Greider, C.W. 1996. Telomerase and cancer: revisiting the telomere hypothesis. Trends. Biochem. Sci. 21: 387–391.
Shay, J.W. and Wright, W.E. 1996. Telomerase activity in human cancer. Curr. Opin. Oncol. 8: 66–71.
Bodnar, A.G., Ouellette, M., Frolkis, M., Holt, S.E., Chiu, C.-P., Morin, G.B. et al. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279: 349–353.
Vaziri, H. and Benchimol, S. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Blol. 8: 279–282.
Martens, U.M., Zijlmans, J.M., Poon, S.S.S., Dragowska, W., Yui, J., Chavez, E.A. et al. 1998. Short telomeres on human chromosome 17p. Nat. Genet. 18: 76–80.
Slagboom, R.E., Droog, S. and Boomsma, D.I. 1994. Genetic determination of telomere size in humans: a twin study of three age groups. Am. J. Hum. Genet. 55: 876–882.
Weng, N.-R., Levine, B.L., June, C.H. and Hodes, R.J. 1995. Human naive and memory T lymphocytes differ in telomeric length and replictive potential. Proc. Natl. Acad. Sci. USA 92: 11091–11094.
Vaziri, H., Dragowska, W., Allsopp, R.C., Thomas, T.E., Harley, C.B. and Lansdorp, P.M. 1994. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc. Natl. Acad. Sci. USA 91: 9857–9860.
Notaro, R., Cimmino, A., Tabarini, D., Rotoli, B. and Luzzatto, L. 1997. In vivo telomere dynamics of human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 94: 13782–13785.
Wynn, R.F., Cross, M.A., Hatton, C., Will, A.M., Lashford, L.S., Dexter, T.M. et al. 1998. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet 351: 178–181.
Wolthers, K.C., Wisman, B.G., Otto, S.A. de Roda Husman, A.M., Schaft, N., de Wolf, F. et al. 1996. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science 274: 1543–1547.
Mohri, H., Bonhoeffer, S., Monard, S., Perelson, A.S. and Ho, D.D. 1998. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science 279: 1223–1227.
Effros, R.B. and Pawelec, G. 1997. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion. Immunol. Today 18: 450–454.
Weng, N.-R., Palmer, L.D., Levine, B.L., Lane, H.C., June, C.H. and Hodes, R.J. 1997. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol. Rev. 160: 43–54.
Lansdorp, P.M., Verwoerd, N.P., van de Rijke, F.M., Dragowska, V., Little, M.-T., Dirks, R.W. et al. 1996. Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 5: 685–691.
Smith, S.H., Brown, M.H., Rowe, D., Callard, R.E. and Beverley, R.C. 1986. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL-1. Immunology 58: 63–70.
Hamann, D., Baars, P.A., Rep, M.H.G., Hooibrink, B., Kerkhof-Garde, S.R., Klein, M.R. et al. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186: 1407–1418.
Tough, D.F. and Sprent, J. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179: 1127–1135.
Palmer, L.D., Weng, N.-R., Levine, B.L., June, C.H., Lane, H.C. and Hodes, R.J. 1997. Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins. J. Exp. Med. 185: 1381–1386.
Trask, B., Van Den Engh, G., Landegent, J. Jansen in de Wai, N., and van der Ploeg, M. 1985. Detection of DNA sequences in nuclei in suspension by in situ hybridization and dual beam flow cytometry. Science 230: 1401–1403.
Trask, B., Van Den Engh, G., Pinkel, D., Mullikin, J., Waldman, P., van Dekken, H et al. 1988. Fluorescence in situ hybridization to interphase cell nuclei in suspension allows flow cytometric analysis of chromosome content and microscopic analysis of nuclear organization. Hum. Genet. 78: 251–259.
van Dekken, H., Arkesteijn, G.J.A., Visser, J.W.M. and Bauman, J.G.J. 1990.Flow cytometric quantification of human chromosome specific repetitive DNA sequences by single and bicoior fluorescent in situ hybridization to lymphocyte interphase nuclei. Cytometry 11: 153–164.
Arkesteijn, G.J.A., Erpelinck, S.L.A., Martens, A.C.M. and Hagenbeek, A. 1995. Chromosome specific DNA hybridization in suspension for flow cytometric detection of chimerism in bone marrow transplantation and leukemia. Cytometry 19: 353–360.
Yu, G. and Blackburn, E.H. 1991. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell 67: 823–832.
Cao, J., Vescio, R.A., Hong, C.H., Kim, A., Lichtenstein, A.K. and Berenson, J.R. 1995. Identification of malignant cells in multiple myeloma bone marrow with immunoglobulin VH gene probes by fluorescent in situ hybridization and flow cytometry. J. Clin. Invest. 95: 964–972.
Zhu, X., Kumar, R., Mandal, M., Sharma, N., Sharma, H.W., Dhingra, U. et al. 1996. Cell cycle-dependent modulation of telomerase activity in tumor cells. Proc. Natl. Acad. Sci. USA 93: 6091–6095.
Nielsen, P.E., Egholm, M., Berg, R.H. and Buchardt, O. 1991. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 254: 1497–1500.
Egholm, M., Buchardt, O., Christensen, L., Behrens, C., Freier, S., Driver, D.A. et al. 1993. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen bonding rules. Nature 365: 566–568.
ZijImans, J.M., Martens, U.M., Poon, S.S.S., Raap, A.K., Tanke, H.J., Ward, R.K. et al. 1997. Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc. Natl. Acad. Sci. USA 94: 7423–7428.
Roosnek, E. and Lanzavecchia, A. 1989. Triggering T cells by otherwise inert hybrid anti-CD3/antitumor antibodies requires encounter with the specific target cell. J. Exp. Med. 170: 297–302.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rufer, N., Dragowska, W., Thornbury, G. et al. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol 16, 743–747 (1998). https://doi.org/10.1038/nbt0898-743
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0898-743
This article is cited by
-
Telomere Length: Implications for Atherogenesis
Current Atherosclerosis Reports (2023)
-
Molecular combing and its application in clinical settings
Molecular Cytogenetics (2022)
-
Quantifying telomeric lncRNAs using PNA-labelled RNA-Flow FISH (RNA-Flow)
Communications Biology (2022)
-
High-throughput STELA provides a rapid test for the diagnosis of telomere biology disorders
Human Genetics (2021)
-
The advancement of telomere quantification methods
Molecular Biology Reports (2021)