Abstract
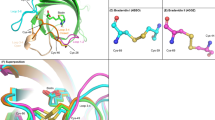
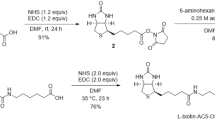
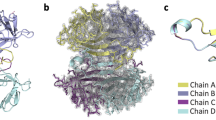
Natural tetrameric streptavidin has two subunit interfaces; one is a strong interface between subunits in a tightly associated dimer, and the other is a weak interface between a pair of such dimers (dimer-dimer interface). To test whether strengthening the weak dimer-dimer interface could provide streptavidin with additional structural stability, covalent crosslinks were introduced between adjacent subunits through the dimer-dimer interface. Specific crosslinking sites were designed by site-directed mutations of His-127 residues that are in close proximity in natural streptavidin. The first and second streptavidin constructs have a disulfide bond and an irreversible covalent bond, respectively, between two Cys-127 residues across the dimer-dimer interface. The third variant is a hybrid tetramer consisting of two different streptavidin species, one having lysine and the other aspartic acid at position 127, which are covalently crosslinked. All streptavidin constructs with intersubunit crosslinks showed higher biotin-binding ability than natural core streptavidin after heat treatment. All of these crosslinked Streptavidins retained bound biotin more stably than natural core streptavidin in guanidine hydrochloride at very acidic pH. These results suggest that the introduction of covalent bonds across the dimer-dimer interface enhances the overall stability of streptavidin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Chalet, L. and Wolf, F.J. 1964. The properties of streptavidin, a biotin-binding protein produced by Streptomycetes . Arch. Biochem. Biophys. 106: 1–5.
Green, N.M. 1975. Avidin Adv. Prot. Chem. 29: 85–133.
Hendrickson, W.A., Pähler, A., Smith, J.L., Satow, Y., Merrit, E.A. and Phizackerley, R.P. 1989. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc. Natl. Acad. Sci. USA 86: 2190–2194.
Weber, P.C., Ohlendorf, D.H., Wendoloski, J.J. and Salemme, F.R. 1989. Structural origins of high-affinity biotin binding to streptavidin. Science 243: 85–88.
Chilkoti, A., Tan, P.H. and Stayton, P.S. 1995. Site-directed mutagenesis studies of the high-affinity streptavidin-biotin complex: Contributions of tryptophan residues 79,108, and 120. Proc. Natl. Acad. Sci. USA 92: 1754–1758.
Sano, T. and Cantor, C.R. 1995. Intersubunit contacts made by tryptophan 120 with biotin are essential for both strong biotin binding and biotin-induced tighter subunit association of streptavidin. Proc. Natl. Acad. Sci. USA 92: 3180–3184.
Chilkoti, A., Schwartz, B.L., Smith, R.D., Long, C.J. and Stayton, P.S. 1995. Engineered chimeric streptavidin tetramers as novel tools for bioseparations and drug delivery. Bio/Technology 11: 1198–1204.
Studier, F.W., Rosenberg, A.M., Dunn, J.J. and Dubendorff, J.W. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185: 60–89.
Sano, T. and Cantor, C.R. 1990. Expression of a cloned streptavidin gene in Escherichia coli . Proc. Natl. Acad. Sci. USA 87: 142–146.
Hofmann, K., Wood, S.W., Brinton, C.C., Montibeller, J.A. and Finn, F.M. 1980. Iminobiotin affinity columns and their application to retrieval of streptavidin. Proc. Natl. Acad. Sci. USA 77: 4666–4668.
Husain, S.S. and Lowe, G. 1968. Evidence for histidine in the active sites of ficin and stem-bromelain. Biochem. J. 110: 53–57.
Husain, S.S. and Lowe, G. 1968. Evidence for the presence of histidine-106 in the active site of papain. Chem. Commun. 1968, 310–311.
Sano, T., Pandori, M.W., Smith, C.L. and Cantor, C.R. 1994. Tighter subunit association of streptavidin upon biotin binding, pp. 21–29 in Advances in biomagnetic separation. Uhlén, M., Homes, E., and Olsvik, Ø (eds.). Eaton Publishing, Natick, MA.
Sano, T., Pandori, M.W., Chen, X., Smith, C.L. and Cantor, C.R. 1995. Recombinant core streptavidins. A minimum-sized core streptavidin has enhanced structural stability and higher accessibility to biotinylated macromolecules. J. Biol. Chem. 270: 28204–28209.
Sayers, J.R., Schmidt, W. and Eckstein, F. 1988. 5′-3′ exonucleases in phospho-rothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 16: 791–802.
Sano, T., Smith, C.L. and Cantor, C.R. 1993. A streptavidin mutant containing a cysteine stretch that facilitates production of a variety of specific streptavidin conjugates. Bio/Technology 11: 201–206.
Staros, J.V., Wright, R.W. and Swingle, D.M. 1986. Enhancement by N-hydroxy-sulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156: 220–222.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular cloning: A laboratory manual, 2nd ed., Cold Spring Harbor Laboratory Press, Plainview, NY.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Wei, R.-D. 1970. Assay of Avidin. Methods Enzymol. 18A: 424–427.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reznik, G., Vajda, S., Smith, C. et al. Streptavidins with intersubunit crosslinks have enhanced stability. Nat Biotechnol 14, 1007–1011 (1996). https://doi.org/10.1038/nbt0896-1007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0896-1007
This article is cited by
-
A monovalent streptavidin with a single femtomolar biotin binding site
Nature Methods (2006)
-
Avidin related protein 2 shows unique structural and functional features among the avidin protein family
BMC Biotechnology (2005)