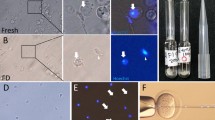
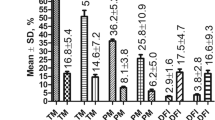
Abstract
Freeze-dried mouse spermatozoa are all motionless and dead in the conventional sense. When injected into oocytes, however, their nuclei can support normal embryonic development even after three month preservation in a dried state. Although the freeze-drying protocol reported here will need further improvement, the results suggest that it may be possible to store the male genomes at room temperature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Polge, C., Smith, A.U., and Parkes, A.S. 1949. Revival of spermatozoa after vitrification and dehydration at low temperature. Nature 164: 666–667.
Smith, A.U. and Polge, C. 1950. Survival of spermatozoa at low temperatures. Nature 166: 668–669.
Polge, C. and Rowson, L.E.A. 1952. Fertilizing capacity of bull spermatozoa after freezing at −79°C. Nature 169: 626–627.
Bunge, R.G., Keettel, W.C., and Sherman, J.K. 1954. Clinical use of frozen semen. Pert. Steril. 5: 520–529.
Watson, P.F. 1990. Artificial insemination and the preservation of semen, pp. 746–869 in Marshall's physiology of reproduction, Vol. 2. Lamming, G.E. (ed.), Churchill Livingstone, London.
Sherman, J.K. 1990. Cryopreservation of human semen, pp. 229–269 in CRC handbook of the laboratory diagnosis and treatment of infertility. Keel, B.A. and Webster B. (eds.), CRC Press, Boca Raton.
Sherman, J.K. 1954. Freezing and freeze-drying of human spermatozoa. Fert. Steril. 5: 357–371.
Bialy, G. and Smith, V.R. 1957. Freeze-drying of bovine spermatozoa. J. Dairy Sci. 40: 739–745.
Nei, T. and Nagase, H. 1961. Attempts to freeze-dry bull spermatozoa. Low Temp. Sci. (SerB) 19: 107–115.
Singh, S.G. and Roy, D.J. 1967. Freeze-drying of bovine semen. Indian J. Vet. Sci. 37: 1–7.
Yushchenko, N.P. 1957. Proof of the possibility of preserving mammalian spermatozoa in a dried state. Proc. Lenin Acad. Agr. Sci. 22: 37–40.
Saacke, R.G. and Almquist, J.O. 1961. Freeze-drying of bovine spermatozoa. Nature 192: 995–996.
Menryman, H.T. 1960. Drying of living mammalian cells. Ann. N.Y. Acad. Sci. 85: 792–739.
Larson, E.V. and Graham, E.F. 1977. Freeze-drying of spermatozoaM, pp. 343–348 in International symposium on freezing biological products, Vol. 36.Cabasso, V.J. and Regamey, R.H, (eds,), Karger S., Basel, Switzerland.
Jeyendran, R.S., Hunter, A.S., and Graham, E.F. 1983. Alteration of seminal proteins during freezing of bovine semen. J. Dairy Sci. 66: 887–891.
Day, J.G. and McLellan, M.R. 1995. Cryopreservation and freeze-drying protocols, vol. 38 of Methods in molecular biology. Humana Press, Totowa, NJ.
Goto, K., Kinioshita, A., Takuma, Y., and Ogawa, K. 1990. Fertilization of bovine oocytes by the injection of immobilized, killed spermatozoa. Vet. Rec. 127: 517–520.
Palermo, G., Joris, H., Devroey, P. and Van Steirteghem, A.C. 1992. Pregnancies after intracytoplasmic injection of a single spermatozoon into an oocyte. Lancet 340: 17–18.
Catt, J. and O'Neill, C. 1995. Manipulation of sperm before intracytoplasmic sperm injection improves fertilization. Fertil. Steril. 64: 1210–1212.
Dozortsev, D., Rybouchkin, A., De Sutter, P., and Dhout, M. 1995. Sperm plasma membrane damage prior to intracytoplasmic sperm injection: a necessary condition for sperm nucleus decondensation. Hum. Reprod. 10: 1960–1964.
Yanagimachi, R. 1997. The mammalian oocyte's response to intracytoplasmic sperm injection, pp. 187–195 in Microscopy of reproduction and development: a dynamic approach. Motta, P.M. (ed.), Antonio Delfino Editore, Rome.
Wakayama, T., Whittingham, D.G., and Yanagimachi, R. 1998. Production of normal offspring from mouse oocytes injected with spermatozoa cryopreserved with or without cryoprotection. J. Reprod. Fert. 112: 11–17.
Uehara, T. and Yanagimachi, R. 1967. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol. Reprod. 15: 467–470.
Katayose, H., Matsuda, J. and Yanagimachi, R. 1992. The ability of dehydrated hamster and human sperm nuclei to develop into pronuclei. Biol. Reprod. 47: 277–284.
Swann, K. 1993. The soluble sperm oscillogen hypothesis. Zygote 1: 273–279.
Kimura, Y., Yanagimachi, R., Kuretake, S., Bortkiewicz, H., Perry, A.C.F., and Yanagimachi, H. 1998. Analysis of mouse egg activation suggests the involvement of sperm perinuclear material. Biol. Reprod. In press.
Schultz, R.M. and Kopf, G.S. 1995. Molecular basis of mammalian egg activation, pp. 21–62 in Current topics in developmental biology, Vol. 30. Petersen, R.A. and Schatten, G. (eds.), Academic Press, San Diego, CA.
Nakagata, N. 1996. Use of Cryopreservation techniques of embryos and spermatozoa for production of transgenic (Tg) mice and for maintenance of Tg mouse lines. Lab. Anim. Sci. 46: 236–238.
Bedford, J.M. and Cooper, H.I. 1974. The occurrence and possible functional significance of -S-S- crosslinks in sperm heads with particular reference to eutherian mammals. J. Exp. Zool. 188: 137–156.
Chatot, C.L., Ziomek, A., Bavister, B.D., Lewis, J.L., and Torres, I. 1989. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 86: 679–688.
Chatot, C.L., Lewis, J.L., Torres, I., and Xiomek, C.A. 1990. Development of 1-cell embryos from different strains of mice in CZB medium. Biol. Reprod. 42: 432–440.
Kimura, Y. and Yanagimachi, R. 1995. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 52: 709–720.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wakayama, T., Yanagimachi, R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol 16, 639–641 (1998). https://doi.org/10.1038/nbt0798-639
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0798-639
This article is cited by
-
Healthy cloned offspring derived from freeze-dried somatic cells
Nature Communications (2022)
-
Production of mouse offspring from zygotes fertilized with freeze-dried spermatids
Scientific Reports (2022)
-
Long-term storage of gametes and gonadal tissues at room temperatures: the end of the ice age?
Journal of Assisted Reproduction and Genetics (2022)
-
Drying and temperature induced conformational changes of nucleic acids and stallion sperm chromatin in trehalose preservation formulations
Scientific Reports (2021)
-
Whole genome integrity and enhanced developmental potential in ram freeze-dried spermatozoa at mild sub-zero temperature
Scientific Reports (2020)