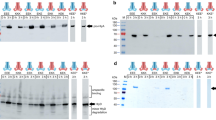
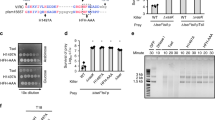
Abstract
Sequence–specific enzymatic cleavage of protein fusions is an important application in recombinant protein technology. We have used the Neisseria type 2 lgA protease (EC 3.4.24.13), produced and secreted by Escherichia coli host cells, for efficiently processing polypeptides at authentic or engineered target sites. In different substrates, the microbial protease specifically cleaves the peptide bond distal to the second Pro residue of the sequence Yaa–Pro–|–Xaa–Pro, where Yaa stands for Pro (or rarely for Pro in combination with Ala, Gly or Thr) and Xaa stands for Thr, Ser or Ala. Highly specific proteolysis has been obtained not only with soluble and purified protein fusions but also with insoluble aggregates derived from cytoplasmic inclusion bodies. The sequence–specificity and simple production of the recombinant IgA protease make it a versatile tool for the in vitro processing of recombinant proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Plant, A.G., Gilbert, J.V., Artenstein, M.S. and Capra, J.D. 1975. Neissrria gonorrhoeae and Neissena meningitidis: Extracellular enzyme cleaves human immunoglobillin A. Science 190: 1103–1105.
Kilian, M., Mestecky, J. and Schrohenloher, R.E. 1979. Pathogenic species of the genus Haemophilus and Streptococcus pneumomiae produce Immunoglobulin A1 protease. Infect. Immun. 26: 143–149.
Mulks, M.H., Plaut, A.G., Feldman, H.A. and Frangionc, B. 1980. IgA proteases of two distinct specificities are released by Neisseria meningitidis . J. Exp. Med. 152: 1442–1447.
Pohlner, J., Halter, R., Beyreuther, K. and Meyer, T.F. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325: 458–462.
Poulsen, K., Brandt, J., Hjorth, J.P., Thogersen, H.C. and Kilian, M. 1989 Cloning and sequencing of the immunoglobulin A1 protease gene (iga) of Haemophilus influenzae Serotype b. Infect. Immun. 57: 3097–3105.
Bachovchin, W.W., Plaut, A.G., Flentke, G.R., Lynch, M. and Kettner, C.A. 1990. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Haemophilus influenzae by peptide prolyl boronic acids. J. Biol. Chem. 265: 3738–3743.
Gilbert, J.V., Plaut, A.G. and Wright, A. 1991. Analysis of the immunoglobulin A protease gene of Streptococcus sanguis . Infect. Immun. 59: 7–17.
Mortensen, S.B. and Kilian, M. 1984. Purification and characterization of an irnmunoglobulin Al protease from Bacerioides melaninogenicus . Infect. Immun. 45: 550–557.
Halter, R., Pohlner, J. and Meyer, T.F. 1984. IgA protease of Neisseria gonorrhoeae: isolation and characterization of the gene and its extracellular product. EMBO J. 3: 1595–1601.
Sung, W.L., Yao, F.-L., Zahab, D.M. and Narang, S.A. 1986. Short synthetic oligodeoxyribonucleotide leader sequences enhance accumulation of human proinsulin synthesized in Escherichia coli . Proc. Natl. Acad. Sci. USA 83: 561–565.
Nagai, K. and Thogersen, H.C. 1984. Generation of β-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli . Nature 309: 810–812.
Germino, J. and Bastia, D. 1984. Rapid purification of a cloned gene product by genetic fusion and site-specific proteolysis. Proc. Natl. Acad. Sci. USA 81: 4692–4696.
Hopp, T.P., Prickett, K.S., Price, V.L., Libby, R.T., March, C.J., Gerretti, D.P., Urdal, D.L., and Conlon, P.J. 1988. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio/Technology 6: 1204–1210.
Nagai, K. and Thogersen, H.C. 1987. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli . Methods Enzymol. 153: 461–481.
Asagoe, Y., Yasukawa, K., Saito, T., Maruo, N., Miyata, K., Kono, T., Miyake, T., Kato, T., Kakidani, H. and Mitani, M. 1988. Human B-cell stimulatory factor-2 expressed in Escherichia coii . Bio/Technology 6: 806–809.
Meyer, T.F., Halter, R. and Pohlner, J. 1987. Mechanism of extracellular secretion of an IgA protease by gram-negative host cells, p. 1271–1281. In: Recent Advances in Mucosal Immunology, Vol. 216B. McGheeJ. R., Mestecky, J., Ogra, P. L., and Bienenstock, J. (E.ds.). Plenum Press. New York.
Strcbel, K., Beck, E., Strohmaier, K.J. and Schaller, H. 1986. Characterization of Foot-and-Mouth Disease virus gene products with antisera against bacterially synthesized fusion proteins. J. Virol. 57: 983–991.
Halter, R., Pohlner, J. and Meyer, T.F. 1989. Mosaic-like organization of IgA protease genes in Neisseria gonorrhoeae: generated by horizontal genetic exchange in vivo . EMBO J. 8: 2737–2744.
Pohlner, J., Maercker, C., Apfel, H. and Meyer, T.F. 1991. Molecular analyses of Neisseria and Haemophilus IgA proteases, p. 567–570. In: Frontiers of Mucosal Immunology, Vol. 1. Tsuchiya, M., Nagura, H. Hibi, T., and More, I. (Eds.). Excerpta Medica, Amsterdam-New York-Oxford.
Sukhatme, V.P., Sizer, K.C., Vollmer, A.C., Hunkapillar, I. and Parnes, J.R. 1985. The T cell differentiation antigen Leu-2/T8 is homologous to irnmunoglobulin and I cell receptor variable regions. Cell 40: 591–597.
Klauser, T., Pohlner, J. and Meyer, T.F. 1990. Extracellular transport of cholera toxin B subunit using Neisseria IgA protease β-domairi: conformation-dependent outer membrane translocation. EMBO J. 9: 1991–1999.
Klauser, T., Pohlner, J. and Meyer, T.F. 1992. Selective extracellular release of cholera toxin B subunit by Escherichia coli: Dissection of Neisseria Igaβ -mediated outer membrane transport. EMBO J. In press.
Wood, S.G. and Burton, J. 1991. Synthetic peptide substrates for the immunoglobulin A1 protease from Neisseria gonorrhoeae (Type 2). Infect. Immun. 59: 1818–1822.
Mollay, C., Vilas, V., Hutticher, A. and Kreil, G. 1986. Isolation of a dipeptidyl aminopeptidase, a putative processing enzyme, from skin secretion of Xenopus laevis . Eur.J. Biochem. 160: 31–35.
Heins, J., Welker, P., Schonlein, G., Born, I., Hartrodt, B., Ncubert, K., Tsuru, D. and Barth, A. 1988. Mechanism of prolinc specific proteinases: (I) substrate specificity of dipeptidyl peptidase IV from pig kidney and proline-specific endopeptidase from Flavobacterium meningosepticum . Bio-chim. Biophys. Acta 954: 161–169.
Hennecke, F., Kolmar, H., Bründl, K. and Fritz, H.-J. 1992 The vsr gene product of E. coli K-12 is a strand- and sequence-specific DNA mismatch endonuclease. Nature 353: 776–778.
Remaut, F., Tsao, H. and Fiers, W. 1983. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene 22: 103–113.
Earhart, C.F., Lundrigan, M., Pickett, C.L. and Pierce, J.R. 1979. Escherichia coli K-12 mutants that lack major outer membrane protein a . FEMS Microbiol. Lett. 6: 277–280.
Saiki, R.K., Gelfand, D.H., Slotfel, S., Scharf, S.J., Higuchi, R., Horn, G.T., Mullis, K.B. and Erlich, H.A. 1988. Primer-directed enzvmatic amplification of DNA with a thermostable DNA polymerase. Science 230: 487–494.
Pohlner, J., Meyer, T.F. and Manning, P.A. 1986. Serological properties and processing in Escherichia coli K12 of OmpV fusion Foteins of Vibrio cholerae . Mol. Gen. Genet. 205: 501–506.
Remaut, E., Stanssens, P. and Fiers, W. 1981. Plasmid vectors for high-efficiency expression controlled by the: p1 promoter of coliphage lambda. Gene 15 81–93.
Chen, E.Y. and Seeburg, P.H. 1985. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA 4: 165–170.
Hunkapiller, M.W., Lujan, E., Ostrander, F. and Hood, L.E. 1983. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 91: 227–236.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pohlner, J., Klauser, T., Kuttler, E. et al. Sequence–Specific Cleave of Protein Fusion Using a Recombinant Neisseria Type 2 IgA Protease. Nat Biotechnol 10, 799–804 (1992). https://doi.org/10.1038/nbt0792-799
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0792-799