Abstract
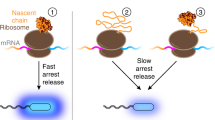
The accumulation of newly synthesized polypeptide chains expressed from cloned genes as non native aggregates has become an important factor in the recovery of such proteins. Studies of both the refolding of denatured proteins in vitro, and of in vivo folding and maturation pathways, indicate that aggregates derive from specific partially folded intermediates and not from mature native, or fully unfolded proteins. The aggregation process in both homologous and heterologous cytoplasms may be driven by partial intracellular denaturation of intermediates, for example by high temperature, or by the absence of a critical factor—prosthetic group, sub-unit, chaperone—during the maturation process. All of these processes appear to be highly specific and subject to modification by genetic engineering of the intermediates, or alteration of their environment. This requires appreciation of the properties of such intermediates as distinct from the native states.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Simons, G., Remaut, E., Allet, B., Devos, R. and Fiers, W. 1984. High-level expression of human interferon gamma in Escherichia coli under control of the p1 promoter of bacteriophage lambda. Gene 28:55–64.
Schoner, E.G., Ellis, L.F. and Schoner, B.E. 1985. Isolation and purification of protein granules from E. coli cells overproducing bovine growth hormone. Bio/Technology 3:151–154.
Taylor, G., Hoare, M., Gray, D.R., and Marston, F.A.O. 1986. Size and density of protein inclusion bodies. Bio/Technology 43:553–557.
Marston, F.A.O. 1986. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem. J. 2403:1–12.
Creighton, T.E. 1978. Experimental studies of protein folding and unfolding. Prog. in Biophys. and Mol. Biol. 333:231–298.
Kim, P.S. and Baldwin, R.L. 1982. Specific intermediates in the folding reactions of small proteins and the mechanism of protein folding pathways. Ann. Rev. Biochem. 51:459–489.
King, J. 1986. Genetic analysis of protein folding pathways. Bio/Technology 4:297–303.
Wright, P.E., Dyson, H.J., and Lerner, R.A. 1988. Conformation of peptide fragments of proteins in aqueous solution: Implications for initiation of protein folding. Biochemistry 27:7167–7175.
Carrell, R.W., Lehmann, H. and Hutchison, H.E. 1966. Haemoglobin Koln (beta-98 valine-methionine): an unstable protein causing inclusion-body anaemia. Nature 210:915–916.
Schneider, R.G., Ueda, S., Alperin, J.B., Brimhall, B., and Jones, R.T. 1969. Hemoglobin Sabine beta 91 (F 7) Leu-Pro. An unstable variant causing severe anemia with inclusion bodies. The New England Journal of Medicine 280:739–745.
Prouty, W.F. and Goldberg, A.L. 1972. Fate of abnormal proteins in E. coli: Accumulation in intracellular granules before catabolism. Nature New. Biol. 240:147–150.
Laemmli, U.K., Beguin, F., Gujer-Kellenberger, G. 1970. A Factor preventing the major head protein of bacteriophage T4 from random aggregation. J. Mol. Biol. 47:69–85.
Hemmingsen, S.M., Woolford, C., van der Vies, S.M., Tilly, K., Dennis, D.T., Georgopoulos, C.P., Hendrix, R.W., and Ellis, R.J. 1988. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature 333:330–334.
Botterman, J. and Zabeau, M. 1985. High-level production of the EcoRI endonuclease under the control of the pL promoter of bacteriophage lambda. Gene 37:229–239.
Gribskov, M. and Burgess, R.R. 1983. Overexpression and purification of the sigma subunit of E. coli RNA polymerase. Gene 26:109–118.
Schoemaker, J.M., Brasnett, A.H., and Marston, F.A.O. 1985. Examination of calf prochymosin accumulation in E. coli.: Disulphide linkages are a structural component of prochymosin-containing inclusion bodies. EMBO J. 4:775–780.
Kane, J.F. and Hartley, D.L. 1988. Formation of recombinant protein inclusion bodies in E. coli. Trends in Biotechnol. 6:95–101.
Pigiet, V.P. and Schuster, B.J. 1986. Thioredoxin-catalyzed refolding of disulphide-containing proteins. Proc. Natl. Acad. Sci. USA 83:7643–7647.
Fahey, R.C., Hunt, J.S., and Windham, G.C. 1977. On the cysteine and cystine content of proteins. Differences between intracellular and extracellular proteins. J. Mol. Evol. 10:155–160.
Rudolph, R., Zettlmeissl, G., and Jaenicke, R. 1979. Reconstitution of lactic dehydrogenase. Noncovalent aggregation vs reactivation. 2. Reactivation of irreversibly denatured aggregates. Biochem. 18:5572–5575.
Zettlmeissl, G., Rudolph, R., and Jaenicke, R. 1979. Reconstitution of lactic dehydrogenase. Noncovalent aggregation vs. reactivation 1. Physical properties and kinetics of aggregation. Biochemistry 18:5567–5571.
Haase-Pettingell, C. and King, J. 1988. Formation of aggregates from a thermolabile in vivo folding intermediate in P22 tailspike maturation: A model for inclusion body formation. J. Biol. Chem. 263:4977–4983.
Anfinsen, C.B., Haber, E., Sela, M., and White, F.H. 1961. The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chains. Proc. Natl. Acad. Sci. USA 47:1309–1314.
Anfinsen, C.B. 1973. Principles that govern the folding of protein chains. Science 181:223–230.
Anson, M.L., and Mirsky, A.E. 1931. The reversibility of protein coagulation. J. of Phys. Chem. 35:185–193.
Perutz, M. 1980. “Unboiling” an egg, p. 14 (from “Discovery”, March 1940). In: Protein Folding. R. Jaenicke (Ed.). Elsevier North-Holland Biomedical Press.
London, J., Skrzynia, C., and Goldberg, M. 1974. Renaturation of E. Coll tryptophanase after exposure to 8 M urea. Evidence for the existence of nucleation centers. Eur. J. Biochem. 47:409–415.
Goldberg, M.E. and Zetina, C.R. 1980. Importance of interdomain interactions in the structure, function and stability of the F1 and F2 domains isolated from the β2 subunit of E. coli tryptophan synthase, p. 469–484. In: Protein Folding. R. Jaenicke (Ed.). Elsevier North-Holland Biomedical Press.
Jaenicke, R. and Rudolph, R. 1980. Folding and association of oligomeric enzymes, p. 525–546. In: Protein Folding. R. Jaenicke (Ed.). Elsevier North-Holland Biomedical Press.
Jaenicke, R. 1987. Folding and association of proteins. Prog. in Biophys. and Mol. Biol. 49:117–237.
Mitraki, A., Betton, J.-M., Desmadril, M. and Yon, J. 1987. Quasi-irreversibility in the unfolding-refolding transition of phosphoglycerate kinase induced by guanidine hydrochloride. Eur. J. Biochem. 163:29–34.
Tandon, S. and Horowitz, P. 1986. Detergent-assisted refolding of guanidinium chloride-denatured rhodanese. The effect of lauryl maltoside. J. Biol. Chem. 261:15615–15681.
Horowitz, P. and Simon, D. 1986. The enzyme rhodanese can be reactivated after denaturation in guanidinium chloride. J. Biol. Chem. 261:13887–13891.
Horowitz, P. and Criscimagna, N.L. 1986. Low concentrations of guanidinium chloride expose apolar surfaces and cause differential perturbation in catalytic intermediates of rhodanese. J. Biol. Chem. 261:15652–15658.
Havel, H.A., Kauffman, E.W., Plaisted, S.M., and Brems, D.N. 1986. Reversible self-association of bovine growth hormone during equilibrium unfolding. Biochemistry 25:6533–6538.
Brems, D.N., Plaisted, S.M., Kauffman, E.W., and Havel, H.A. 1986. Characterization of an associated equilibrium folding intermediate of bovine growth hormone. Biochemistry 25:6539–6543.
Brems, D.N., Plaisted, S.M., Havel, H.A. and Tomich, C-S.C. 1988. Stabilization of an associated folding and intermediate of bovine growth hormone by site-directed mutagenesis. Proc. Natl. Acad. USA 85:3367–3371.
Brems, D.N., Plaisted, S.M., Kaufmann, E.W., Lund, M., and Lehrmann, S.R. 1987. Helical formation in isolated fragments of bovine growth hormone. Biochemistry 26:7774–7778.
Brems, D.N. 1988. Solubility of different folding conformers of bovine growth hormone. Biochemistry 27:4541–4546.
Goldberg, M.E. 1985. The second translation of the genetic message: protein folding and assembly. Trends in Biochemical Sciences 10:388–391.
Goldenberg, D.P., Smith, D.H., and King, J. 1983. Genetic analysis of the folding pathway for the tail spike protein of phage P22. Proc. Nat Acad. Sci. USA 80:7060–7064.
Goldenberg, D. and King, J. 1982. Trimeric intermediate in the in vivo folding and subunit assembly of the tail spike endhorhamnosidase ol bacteriophage P22. Proc. Natl. Acad. Sci. USA 79:3403–3407.
Goldenberg, D.P., Berget, P.B., and King, J. 1982. Maturation of the tailspike endorhamnosidase of Salmonella phage P22. J. Biol. Chem. 257:7864–7871.
Goldenberg, D. and King, J. 1981. Temperature-sensitive mutants blocked in the folding or subunit assembly of bacteriophage P22 tail spike protein. II. Active mutant proteins matured at 30°C. J. Mol. Biol. 145:633–651.
Sturtevant, J.M., Yu, M-H., Hasse-Pettingell, C. and King, J. 1989. Thermostability of temperature-sensitive mutants of P22 tailspike protein. J. Biol. Chem. In Press.
Fane, B.A. 1988. Genetic analysis of folding and maturation defects in the P22 tailspike endorhamnosidase. Ph.D. Thesis, Massachusetts Institute of Technology.
Mizukami, T., Komatsu, Y., Hosoi, N., Hoh, S., and Oka, T. 1986. Production of active human interferon-beta in E. coli. I. Preferential production by lower culture temperature. Biotechnology Letts 8:605–610.
Schein, C.H. and Noteborn, M.H.M. 1988. Formation of soluble recombinant proteins in E. colt, is favored by lower growth temperature. Bio/Technology 6:291–294.
Takagi, H., Morinaga, Y., Tsuchiya, M., Ikemura, H., and Inouye, M. 1988. Control of folding of proteins secreted by a high expression secretion vector, pIN-III-ompA: 16-fold increase in production of active subtilisin E in E. coli. Bio/Technology 6:948–950.
Ikemura, H., Takagi, H., and Inouye, M. 1987. Requirement of prosequence for the production of active subtilisin E in E. coli. J. Biol. Chem. 262:7859–7864.
Copeland, C.S., Doms, R.W., Bolzeau, E.M., Webster, R.G., and Helenius, A. 1986. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J. Cell. Biol. 103:1179–1191.
Gething, M.-J., McCammon, K. and Sambrook, J. 1986. Expression of wild-type and mutant forms of influenza hemagglutinin: The role of folding in intracellular transport. Cell 46:939–950.
Munro, S. and Pelham, H.R.B. 1986. An Hsp70-like protein in the ER: Identity with the 78 kD glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46:291–300.
Kozutsumi, Y., Segal, M., Normington, K., Gething, M-J., and Sambrook, J. 1988. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332:462–464.
Pelham, H.R.B. 1986. Speculations on the functions of the major heat-shock and glucose-regulated proteins. Cell 46:959–961.
Teipel, J.W. and Koshland, D.E. Jr. 1971. Kinetic aspects of conformational changes in proteins. I. Rate of regain of enzyme activity from denatured proteins. Biochemistry 10:792–805.
Rudolph, R., Gerschitz, J. and Jaenicke, R. 1978. Effect of zinc (II) on the refolding and reactivation of liver alcohol dehydrogenase. Eur. J. Biochem. 87:601–606.
Leutzinger, Y. and Beychock, S. 1981. Kinetics and mechanism of heme-induced refolding of human aipha-globin. Proc. Natl. Acad. Sci. USA 78:780–784.
Martel, A. and Garel, J.R. 1984. Renaturation of the allosteric phosphofructokinase from E. coli. J. Biol. Chem. 259:4917–4921.
Brambl, R. and Plesofsky-Vig, N. 1986. Pantothenate is required in Neurospora crassa for assembly of subunit peptides of cytochrome c oxidase and ATPase/ATP synthase. Proc. Natl. Acad. Sci. USA 83:3644–3648.
Binkowski, G., and Simon, L.D. 1983. Host functions that affect T4 reproduction, p. 342–350. In: Bacteriophage T4 (C. K. Matthews, E. M. Kutter, G. Mosig, and P. B. Berget (Eds.). Am. Soc. Microbiol., Wash. D.C.
Georgopoulos, C., Tilly, K., and Casjens, S. 1983. Lambdoid phage head assembly, p. 279–304. In: Lambda II Hendrix, R. W., Roberts, J. W., Stahl, F. W., and Weisberg, R. A. (Eds.). Cold Spring Harbor, New York.
King, J. and Laemmli, U.K. 1971. Polypeptides of the tail fibers of bacteriophage T4. J. Mol. Biol. 62:465–477.
Prevelige, P., Thomas, D. and King, J. 1988. Scaffolding protein regulates the polymerization of P22 coat subunits into icosahedral shells in vitro. J. Mol. Biol. 202:743–757.
Bochkareva, E.S., Lissin, N.M. and Girshovich, A.S. 1988. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature 336:254–257.
Randall, L.L., Hardy, S.J.S., and Thom, J.R. 1987. Export of protein: A biochemical view. Ann. Rev. Microbiol. 41:507–541.
Meyer, D.I. 1988. Preprotein conformation: The year's major theme in translocation studies. Trends Biochem. Sci. 13:471–474.
Collier, D.N., Bankaitis, V.A., Weiss, J.B., and Bassford, P.J. Jr. 1988. The anti-folding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell 53:273–283.
Glenner, G.G. 1981. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). New England Journal of Medicine 302:1283–1292.
Kirschner, D.A., Inouye, H., Duffy, L.K., Sinclair, A., Lind, M., and Selkoe, D.J. 1987. Synthetic peptide homologous to beta protein from Alzheimer disease forms amyloid-like fibrils in vitro. Proc. Natl. Acad. Sci. USA. 84:6953–6957.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mitraki, A., King, J. Protein Folding Intermediates and Inclusion Body Formation.. Nat Biotechnol 7, 690–697 (1989). https://doi.org/10.1038/nbt0789-690
Issue Date:
DOI: https://doi.org/10.1038/nbt0789-690
This article is cited by
-
Construction of a constitutively active type III secretion system for heterologous protein secretion
Applied Microbiology and Biotechnology (2023)
-
Recombinant production of ESAT-6 antigen in thermoinducible Escherichia coli: the role of culture scale and temperature on metabolic response, expression of chaperones, and architecture of inclusion bodies
Cell Stress and Chaperones (2019)
-
Rational Design of Multiple TB Antigens TB10.4 and TB10.4-Ag85B as Subunit Vaccine Candidates Against Mycobacterium Tuberculosis
Pharmaceutical Research (2010)
-
The optimization of in vitro high-throughput chemical lysis of Escherichia coli. Application to ACP domain of the polyketide synthase ppsC from Mycobacterium tuberculosis
Journal of Structural and Functional Genomics (2010)