Abstract
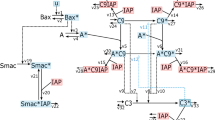
Caspases (cysteine-containing aspartate-specific proteases) are at the core of the cell's suicide machinery. These enzymes, once activated, dismantle the cell by selectively cleaving key proteins after aspartate residues. The events culminating in caspase activation are the subject of intense study because of their role in cancer, and neurodegenerative and autoimmune disorders. Here we present a mechanistic mathematical model, formulated on the basis of newly emerging information, describing key elements of receptor-mediated and stress-induced caspase activation. We have used mass-conservation principles in conjunction with kinetic rate laws to formulate ordinary differential equations that describe the temporal evolution of caspase activation. Qualitative strategies for the prevention of caspase activation are simulated and compared with experimental data. We show that model predictions are consistent with available information. Thus, the model could aid in better understanding caspase activation and identifying therapeutic approaches promoting or retarding apoptotic cell death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Evan, G. & Littlewood, T. A matter of life and cell death . Science 281, 1317–1722 (1998).
Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462 ( 1995).
Haass, C. Apoptosis. Dead end for neurodegeneration? Nature 399 , 204–207 (1999).
Ashkenazi, A. & Dixit, V.M. Death receptors: signaling and modulation . Science 281, 1305–1308 (1998).
Adams, J.M. & Cory, S. The Bcl-2 protein family: arbiters of cell survival. Science 281, 1322– 1326 (1998).
Thornberry, N.A. & Lazebnik, Y. Caspases: enemies within. Science 281, 1312– 1316 (1998).
Green, D.R. & Reed, J.C. Mitochondria and apoptosis. Science 281, 1309–1312 (1998).
Smith, C.A., Farrah, T. & Goodwin, R.G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76, 959–962 (1994).
Tartaglia, L.A., Ayres, T.M., Wong, G.H. & Goeddel, D.V. A novel domain within the 55 kd TNF receptor signals cell death. Cell 74, 845–853 (1993).
Nagata, S. Apoptosis by death factor. Cell 88, 355– 365 (1997).
Huang, B., Eberstadt, M., Olejniczak, E.T., Meadows, R.P. & Fesik, S.W. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 384, 638–641 (1996).
Chinnaiyan, A.M., O'Rourke, K., Tewari, M. & Dixit, V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, 505– 512 (1995).
Boldin, M.P. et al. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 270, 7795–7798 ( 1995).
Boldin, M.P. et al. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J. Biol. Chem. 270, 387–391 (1995).
Muzio, M., Stockwell, B.R., Stennicke, H.R., Salvesen, G.S. & Dixit, V.M. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273, 2926– 2930 (1998).
Salvesen, G.S. & Dixit, V.M. Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. USA 96, 10964–10967 (1999).
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997).
Yang, J. et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275, 1129– 1132 (1997).
Kluck, R.M., Bossy-Wetzel, E., Green, D.R. & Newmeyer, D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275, 1132– 1136 (1997).
Simpson, N.H., Singh, R.P., Perani, A., Goldenzon, C. & Al-Rubeai, M. In hybridoma cultures, deprivation of any single amino acid leads to apoptotic death, which is suppressed by the expression of the bcl-2 gene. Biotecnol. Bioeng. 59, 90– 98 (1998).
Goswami, J., Sinskey, A.J., Steller, H., Stephanopoulos, G.N. & Wang, D.I. Apoptosis in batch cultures of Chinese hamster ovary cells. Biotechnol. Bioeng. 62, 632–640 (1999).
Sanfeliu, A. & Stephanopoulos, G. Effect of glutamine limitation on the death of attached Chinese hamster ovary cells. Biotechnol. Bioeng. 64, 46–53 ( 1999).
Deveraux, Q.L., Takahashi, R., Salvesen, G.S. & Reed, J.C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388, 300–304 ( 1997).
Deveraux, Q.L. et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17, 2215–2223 (1998).
Seshagiri, S. & Miller, L.K. Baculovirus inhibitors of apoptosis (IAPs) block activation of Sf- caspase-1. Proc. Natl. Acad. Sci. USA 94, 13606–13611 ( 1997).
Irmler, M. et al. Inhibition of death receptor signals by cellular FLIP. Nature 388, 125–126 ( 1997).
Shu, H.B., Halpin, D.R. & Goeddel, D.V. Casper is a FADD- and caspase-related inducer of apoptosis . Immunity 6, 751–763 (1997).
Koseki, T., Inohara, N., Chen, S. & Nunez, G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc. Natl. Acad. Sci. USA 95, 5156–5160 (1998).
Amarante-Mendes, G.P. et al, Anti-apoptotic oncogenes prevent caspase-dependent and independent commitment for cell death. Cell Death Differ. 5, 298–306 (1998).
Reed, J.C. Double identity for proteins of the Bcl-2 family. Nature 387, 773–776 (1997).
Newton, K. & Strasser, A. The Bcl-2 family and cell death regulation. Curr. Opin. Genet. Dev. 8, 68 –75 (1998).
Hsu, Y.T., Wolter, K.G. & Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 94, 3668–3672 (1997).
Miyashita, T. & Reed, J.C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995).
Zhang, J., Cado, D., Chen, A., Kabra, N.H. & Winoto, A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 ( 1998).
Yeh, W.C. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998).
Ona, V.O. et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature 399, 263–267 (1999).
Davidson, F.F. & Steller, H. Blocking apoptosis prevents blindness in Drosophila retinal degeneration mutants. Nature 391, 587–591 ( 1998).
Acknowledgements
This work was supported by the Swiss Priority Program in Biotechnology (SPP BioTech).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fussenegger, M., Bailey, J. & Varner, J. A mathematical model of caspase function in apoptosis. Nat Biotechnol 18, 768–774 (2000). https://doi.org/10.1038/77589
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/77589
This article is cited by
-
Optogenetic induction of caspase-8 mediated apoptosis by employing Arabidopsis cryptochrome 2
Scientific Reports (2023)
-
Computational modeling of DLBCL predicts response to BH3-mimetics
npj Systems Biology and Applications (2023)
-
A review on cell damage, viability, and functionality during 3D bioprinting
Military Medical Research (2022)
-
Evaluating a therapeutic window for precision medicine by integrating genomic profiles and p53 network dynamics
Communications Biology (2022)
-
BAX and SMAC regulate bistable properties of the apoptotic caspase system
Scientific Reports (2021)