Abstract
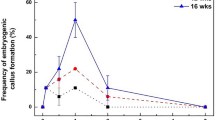
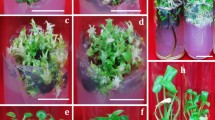
Procedures for the production of a new and highly prolific embryogenic culture system have been developed in cassava. The importance of the basal salts and type of auxin in controlling the development of cassava embryogenic tissues has been demonstrated, with culture on Gresshoff and Doy basal medium in the presence of 4-amino-3,5,6,trichloro-picolinic acid (picloram) inducing the formation of friable embryogenic callus from which highly totipotent embryogenic suspension cultures could be established. Plants have been regenerated from these cultures. The availability of embryogenic suspension cultures is considered to have important implications for the application of genetic transformation and other biotechnologies in the agronomic improvement of cassava.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lynam, J.H. 1993. Potential impact of biotechnology on cassava production in the Third World, pp. 22–30 in Proceedings of the First International Scientific Meeting of the Cassava Biothechnology Network, Cartegena, Colombia, 25-28 August 1992. Roca, W.M. and Thro, A.M. (eds.). Centra Internacional de Agricultura, Cali, Colombia.
Schöpke, C., Franche, C., Bogusz, D., Chavarriaga. P., Fauquet, C., and Beachy, R.N. 1993. Transformation in cassava (Manihot eculenta Crantz), pp. 273–289 in Biotechnology in Agriculture and Forestry, Vol 23. Plant Protoplasts and Genetic Engineering IV. Springer-Verlag, Berlin.
Fauquet, M. and Henshsaw, G.G. 1993. CBN Steering Committee Report, pp. 493–494 in Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network, Cartegena, Colombia, 25-28 August 1992. Roca, W.M. and Thro, A.M. (eds.). Centra Internacional de Agricultura, Cali, Colombia.
Stamp, J.A. and Henshaw, G.G. 1982. Somatic embryogenesis in cassava. Zeitschrift fur Planzenphysiologie 105: 183–187.
Stamp, J.A. and Henshaw, G.G. 1987. Somatic embryogenesis from clonal leaf material of cassava. Ann. of Botany 59: 45–50.
Stamp, J.A. and Henshaw, G.G. 1987. Secondary somatic embryogenesis and plant regeneration in cassava. Plant Cell Tissue and Organ Culture 10: 227–233.
Szabados, L., Hoyos, R., and Roca W. 1987. In vitro somatic embryogenesis and plant regeneration of cassava. Plant Cell Reports 6: 248–251.
Taylor, N.J. and Henshaw, G.G. 1993. The induction of somatic embryogenesis in fifteen African and one South American cassava cultivars, pp.134–139 in Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network. Cartegena, Colombia, 25-28 August 1992. Roca, W.M. and Thro, A.M. (eds.). Centra Internacional de Agricultura, Cali, Colombia.
Raemakers, C.J.J.M., Amati, M., Staritsky, G., Jacobsen, E., and Visser, R.G.F. 1993. Cyclic somatic embryogenesis and plant regeneration in cassava. Ann. of Botany 71: 289–294.
Mathews, H., Schopke, C., Carcamo, R., Chavarriaga, P., Fauquet, C., and Beachy, R.N. 1993. Improvement of somatic embryogenesis and plant recovery in cassava. Plant Cell Reports 12: 328–333.
Vasil, L. 1995. Cellular and molecular improvement of cereals, pp. 5–18 in Current Issues in Plant Molecular and Cellular Biology. Proceedings of the VIIIth Interantional Congress on Plant Tissue and Cell Culture, Florence, Italy, 12-17 June 1994. Terzi, M. et al (eds.). Kluwer Adacemic Publishers, Dordrecht, The Netherlands.
Christou, P. 1992. Genetic transformation of crop plants using microprojectile bombardment. The Plant Journal 2: 275–281.
Vasil, I. and Vasil, V. 1992. Advances in cereal protoplast research. Physiol. Plant. 85: 279–283.
Schöpke, C., Taylor, N., Carcamo, R., Konan, N.K., Marmey, P., Henshaw, G.G., Beachy, R.N., and Fauquet, C. 1996. Regeneration of transgenic cassava plants (Manihot esculenta Crantz) from microbombarded embryogenic suspension cultures. Nature Biotechnology 14: xxx–xxx.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Gresshoff, P. and Doy, C. 1974. Derivation of a haploid cell line from Vitis vinifera and the importance of the stage of meiotic development of anthers for haploid culture of this and other genera. Z. Pflanzenphysiol. 73: 132–141.
Schenk, R. and Hildebrandt, A. 1972. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 50: 199–204.
Hunold, R., Bronner, R., and Hahne, G. 1994. Early events in microprojectile bombardment: cell viability and particle location. The Plant Journal 5: 593–604.
Smith, S. and Street, H. 1974. The decline of embryogenic potential as callus and suspension cultures of carrot are serially subcultured. Ann. of Botany 38: 223–241.
Armstrong, C. and Green, C. 1985. Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 164: 207–214.
Novak, F., Afza. R., Van Duren, M., Perea-Dallos, M., Conger, B., and Tang Xiaolang. 1989. Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA and AAA) and cooking (ABB) bananas (Musa spp.). Bio/Technology 7: 154–156.
McWilliam, A., Smith, S., and Street, H. 1974. The origin and development of embryoids in suspension cultures of carrot (Dacus carota). Ann. of Botany 38: 243–250.
Anthony, P., Davey, M., Power, J., and Lowe, K. 1995. An improved protocol for the culture of cassava leaf protoplasts. Plant Cell Tissue and Organ Culture 42: 299–302.
Widholm, J. 1972. The use of fluorescein diacetate and phensafranine for determining viability of cultured plant cells. Stain Technol. 47: 189–194.
Chu, C., Wang, C., Sun, C., Hsu, C., Yin, K., Chu, C., and Bi, F. 1975. Establishment of an efficient medium for anther culture of rice, through comparative experiments on nitrogen sources. Scientia Sinic. 18: 659–668.
Nitsch, J. and Nitsch, C. 1969. Haploid plants from pollen grains. Science 163: 85–87.
Lloyd, G. and McCowan, B. 1981. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Int. Prop. Soc. Proc. 30: 593–604.
Gamborg, O., Miller, R., and Ojima, K. 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taylor, N., Edwards, M., Kiernan, R. et al. Development of friable embryogenic callus and embryogenic suspension culture systems in cassava (Manihot esculenta Crantz). Nat Biotechnol 14, 726–730 (1996). https://doi.org/10.1038/nbt0696-726
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0696-726