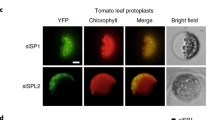
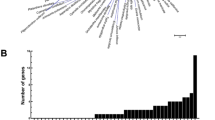
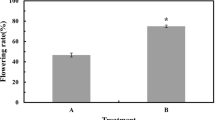
Abstract
Polyamines, ubiquitous organic aliphatic cations, have been implicated in a myriad of physiological and developmental processes in many organisms, but their in vivo functions remain to be determined. We expressed a yeast S-adenosylmethionine decarboxylase gene (ySAMdc; Spe2) fused with a ripening-inducible E8 promoter to specifically increase levels of the polyamines spermidine and spermine in tomato fruit during ripening. Independent transgenic plants and their segregating lines were evaluated after cultivation in the greenhouse and in the field for five successive generations. The enhanced expression of the ySAMdc gene resulted in increased conversion of putrescine into higher polyamines and thus to ripening-specific accumulation of spermidine and spermine. This led to an increase in lycopene, prolonged vine life, and enhanced fruit juice quality. Lycopene levels in cultivated tomatoes are generally low, and increasing them in the fruit enhances its nutrient value. Furthermore, the rates of ethylene production in the transgenic tomato fruit were consistently higher than those in the nontransgenic control fruit. These data show that polyamine and ethylene biosynthesis pathways can act simultaneously in ripening tomato fruit. Taken together, these results provide the first direct evidence for a physiological role of polyamines and demonstrate an approach to improving nutritional quality, juice quality, and vine life of tomato fruit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
US Department of Agriculture. Agricultural Statistics, pp 141–175 (US Government Printing Press, Washington DC, 1991).
Arumuganathan, K. & Earle, E. Nuclear DNA content of some important plant species. Plant Mol. Biol. Reptr. 9, 208–218 (1991).
Giovannoni, J. Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 725–749 (2001).
DellaPenna, D. Nutritional genomics: manipulating plant micronutrients to improve human health. Science 285, 375–379 (1999).
Oeller, P.W., Wong, L.M., Taylor, L.P., Pike, D.A. & Theologis, A. Reversible inhibition of tomato fruit senescence by antisense 1-aminocyclopropane-1-carboxylate synthase. Science 254, 427–439 (1991).
Fluhr, R. & Mattoo, A.K. Ethylene—biosynthesis and perception. Crit. Rev. Plant Sci. 15, 479–523 (1996).
Hamilton, A., Lycett, G. & Grierson, D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 346, 284–287 (1990).
Wilkinson, J.Q. et al. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat. Biotechnol. 15, 444–447 (1997).
Galston, A.W. & Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 94, 406–410 (1990).
Cohen, S.S. A Guide to the Polyamines. (Oxford Univ. Press, New York, 1998).
Cassol, T. & Mattoo, A.K. Do polyamines and ethylene interact to regulate plant growth, development and senescence? In Molecular Insights in Plant Biology (eds Nath, P., Mattoo, A., Ranade, S.R. & Weil, J.H.) (Oxford-IBH, New Delhi, 2002, in press).
Lester, G.E. Polyamines and their cellular anti-senescence properties in honey dew muskmelon fruit. Plant Sci. 160, 105–112 (2000).
Noh, E.W. & Minocha, S.C. Expression of a human S-adenosylmethionine decarboxylase cDNA in transgenic tobacco and its effects on polyamine biosynthesis. Transgenic Res. 3, 26–35 (1994).
Hamill, J.D. et al. Over-expressing a yeast ornithine decarboxylase gene in transgenic roots of Nicotiana rustica can lead to enhanced nicotine accumulation. Plant Mol. Biol. 15, 27–38 (1990).
DeScenzo, R.A. & Minocha, S.C. Modulation of cellular polyamines in tobacco by transfer and expression of mouse ornithine decarboxylase cDNA. Plant Mol. Biol. 22, 113–127 (1993).
Kumar, A., Taylor, M.A., Arif, S.A.M. & Davies, H.V. Potato plants expressing antisense and sense S-adenosylmethionine decarboxylase (SAMDC) transgenes show altered levels of polyamines and ethylene: antisense plants display abnormal phenotypes. Plant J. 9, 147–158 (1996).
Pedros, A.R. et al. Manipulation of S-adenosylmethionine decarboxylase activity in potato tubers—an increase in activity leads to an increase in tuber number and a change in tuber size distribution. Planta 209, 153–160 (1999).
Kashiwagi, K., Taneja, S.K., Liu, T.Y., Tabor, C.W. & Tabor, H. Spermidine biosynthesis in Saccharomyces cerevisiae. Biosynthesis and processing of a proenzyme form of S-adenosylmethionine decarboxylase. J. Biol. Chem. 265, 22321–22328 (1990).
Deikman, J., Klone, R. & Fischer, R.L. Organization of ripening and ethylene regulatory regions in a fruit-specific promoter from tomato (Lycopersicon esculentum). Plant Physiol. 100, 2013–2017 (1992).
Takada, N. & Nelson, P. New consistency method for tomato products—the precipitate weight ratio. J. Food Sci. 48, 1460–1462 (1983).
Beecher, G.R. Nutrient content of tomatoes and tomato products. Proc. Soc. Exp. Biol. Med. 218, 98–100 (1998).
Shi, J. & Le Maguer, M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 20, 293–334 (2000).
Van den Berg, H. et al. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J. Sci. Food Agric. 80, 880–912 (2000).
Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J. Natl. Cancer Inst. 91, 317–331 (1999).
Djuric, Z. & Powell, L.C. Antioxidant capacity of lycopene-containing foods. Intl. J. Food Sci. Nutr. 52, 143–149 (2001).
Thompson, A.E., Tomes, M.L., Wann, E.V., McCollum, J.P. & Stoner, A.K. Characterization of Crimson tomato fruit color. Proc. Amer. Soc. Hort. Sci. 86, 610–616 (1965).
Bramley, P. The regulation and genetic manipulation of carotenoid biosynthesis in tomato fruit. Pure Appl. Chem. 69, 2159–2162 (1997).
Fraser, P.D. et al. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc. Natl. Acad. Sci. USA 99, 1092–1097 (2002).
Alba, R., Cordonnier-Pratt, M.M. & Pratt, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 123, 363–370 (2000).
Schuch, W. et al. Fruit quality characteristics of transgenic tomato fruit with altered polygalacturonase activity. HortScience 26, 1517–1520 (1991).
Kramer, M. et al. Postharvest evaluation of transgenic tomatoes with reduced levels of polygalacturonase: processing firmness, and disease resistance. Postharvest Biol. Technol. 1, 241–255 (1992).
Thakur, B.R., Singh, R.K., Tieman, D.M. & Handa, A.K. Quality of processed tomato products from transgenic tomato fruits with reduced levels of pectin methylesterase activity. J. Food Sci. 61, 245–248 (1996).
Watson, C.F., Zeng, L. & DellaPenna, D. Reduction of tomato polygalacturonase β-subunit expression effects pectin solubilization and degradation during fruit ripening. Plant Cell 6, 1623–1634 (1994).
Hua, J. & Meyerowitz, E.M. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94, 261–271 (1998).
Pennarrubia, L., Aguilar, M., Margossian, L. & Fischer, R.L. An antisense gene stimulates ethylene hormone production during tomato fruit ripening. Plant Cell 4, 681–687 (1992).
Giovannoni, J.J., DellaPenna, D., Bennett, A.B. & Fischer, R.L. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell 1, 53–63 (1989).
De Block, M., Herreraestrella, L., Van Montagu, M., Schell, J. & Zambryski, P. Expression of foreign genes in regenerated plants and in their progeny. EMBO J. 3, 1681–1689 (1984).
Zambryski, P., Joos, H., Genetello, C., Leemans, J. & van Montagu, M. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 2, 2143–2150 (1983).
Tieman, D.M., Harriman, R.W., Ramamohan, G. & Handa, A.K. An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. Plant Cell 4, 667–679 (1992).
Murray, M.G. & Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8, 4321–4325 (1980).
Li, N., Parsons, B., Liu, D. & Mattoo, A.K. Accumulation of wound-inducible ACC synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol. Biol. 18, 477–487 (1992).
Kiss, T., Kiss, M. & Solymosy, F. Nucleotide sequence of a 25S rRNA gene from tomato. Nucleic Acids Res. 17, 796–796 (1989).
Minocha, S.C., Minocha, R. & Robie, C.A. High-performance liquid chromatographic method for the determination of dansyl-polyamines. J. Chromatogr. 511, 177–183 (1990).
Wellburn, A.R. The spectral determination of chlorophyll-A and chlorophyll-B, as well as total carotenoids using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144, 301–313 (1994).
Acknowledgements
We thank Herbert Tabor, Bob Fischer, and Gad Galili for gifts of yeast SAM decarboxylase gene, E8 promoter, and pCD vector, respectively; Aref Abdul-Baki for help with field tests; Zhiping Deng and Tatsiana Datesenka for help in processing data; and James Anderson, Marvin Edelman, Santosh Misra, and Mark Tucker for a careful reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mehta, R., Cassol, T., Li, N. et al. Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine life. Nat Biotechnol 20, 613–618 (2002). https://doi.org/10.1038/nbt0602-613
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0602-613
This article is cited by
-
Polyamines: The Gleam of Next-Generation Plant Growth Regulators for Growth, Development, Stress Mitigation, and Hormonal Crosstalk in Plants—A Systematic Review
Journal of Plant Growth Regulation (2023)
-
Roles of Polyamines in Growth and Development of the Solanaceous Crops Under Normal and Stressful Conditions
Journal of Plant Growth Regulation (2023)
-
Quality trait improvement in horticultural crops: OMICS and modern biotechnological approaches
Molecular Biology Reports (2023)
-
Revisiting the Role of Polyamines in Plant Growth and Abiotic Stress Resilience: Mechanisms, Crosstalk, and Future Perspectives
Journal of Plant Growth Regulation (2023)
-
Exogenously applied spermidine alleviates hypoxia stress in Phyllostachys praecox seedlings via changes in endogenous hormones and gene expression
BMC Plant Biology (2022)