Abstract
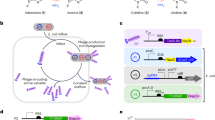
The thymidine kinase (TK) genes from herpes simplex virus (HSV) types 1 and 2 were recombined in vitro with a technique called DNA family shuffling. A high-throughput robotic screen identified chimeras with an enhanced ability to phosphorylate zidovudine (AZT). Improved clones were combined, reshuffled, and screened on increasingly lower concentrations of AZT. After four rounds of shuffling and screening, two clones were isolated that sensitize Escherichia coli to 32-fold less AZT compared with HSV-1 TK and 16,000-fold less than HSV-2 TK. Both clones are hybrids derived from several crossover events between the two parental genes and carry several additional amino acid substitutions not found in either parent, including active site mutations. Kinetic measurements show that the chimeric enzymes had acquired reduced KM for AZT as well as decreased specificity for thymidine. In agreement with the kinetic data, molecular modeling suggests that the active sites of both evolved enzymes better accommodate the azido group of AZT at the expense of thymidine. Despite the overall similarity of the two chimeric enzymes, each contains key contributions from different parents in positions influencing substrate affinity. Such mutants could be useful for anti-HIV gene therapy, and similar directed-evolution approaches could improve other enzyme–prodrug combinations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stemmer, W.P.C. DNA shuffling by random fragmentation and reassembly: in vitro recombination for molecular evolution. Proc. Natl. Acad. Sci. USA 91, 10747–10751 (1994).
Stemmer, W.P.C. Rapid evolution of a protein in vitro by DNA shuffling. Nature 370, 389–391 ( 1994).
Zhang, J.-H., Dawes, G. & Stemmer, W.P.C. Directed evolution of a fucosidase from a galactosidase by DNA shuffling and screening. Proc. Natl. Acad. Sci. USA 94, 4504–4509 (1997).
Crameri, A., Whitehorn, E.A., Tate, E. & Stemmer, W.P.C. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat. Biotechnol. 14, 315– 319 (1996).
Buchholz, F., Angrand, P.-O. & Stewart, A.F. Improved properties of FLP recombinase evolved by cycling mutagenesis. Nat. Biotechnol. 16, 657–662 (1998).
Crameri, A., Raillard, S.-A., Bermudez, E. & Stemmer, W.P.C. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature 391, 288– 291 (1998).
Kumamaru, T., Suenaga, H., Mitsuoka, M., Watanabe, T. & Furukawa, K. Enhanced degradation of polychlorinated biphenyls by directed evolution of biphenyl dioxygenase. Nat. Biotechnol. 16, 663–666 ( 1998).
Hirsch, M.S., Kaplan, J.C. & D'Aquila, R.T. in Fields virology (eds. Fields, B.N., Knipe, D.M. & Howley, P.M.) 431–466 (Lippincott-Raven, Philadelphia, 1996).
Martin, L.-A. & Lemoine, N.R. Direct cell killing by suicide genes. Cancer Metastasis Rev. 15, 301– 316 (1996).
Guettari, N., Loubiere, L., Brisson, E. & Klatzmann, D. Use of herpes simplex virus thymidine kinase to improve the antiviral activity of zidovudine. Virology 235, 398– 405 (1997).
Drake, R.R. et al. Metabolism and activities of 3´-azido-2´,3´-dideoxythymidine and 2´,3´-didehydro-2´,3´-dideoxythymidine in herpesvirus thymidine kinase transduced T-lymphocytes. Antiviral Res. 35, 177–185 (1997).
Gentry, G.A. Viral thymidine kinases and their relatives. Pharmacol. Ther. 54, 319–355 (1992).
Munir, K.M., French, D.C. & Loeb, L.A. Thymidine kinase mutants obtained by random sequence selection. Proc. Natl. Acad. Sci. USA 90, 4012–4016 (1993).
Black, M.E. & Loeb, L.A. Identification of important residues within the putative nucleoside binding site of HSV-1 thymidine kinase by random sequence selection: analysis of selected mutants in vitro. Biochemistry 32, 11618–11626 ( 1993).
Black, M.E., Newcomb, T.G., Wilson, H.-M.P. & Loeb, L.A. Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proc. Natl. Acad. Sci. USA 93, 3525–3529 (1996).
Moore, J.C., Jin, H.-M., Kuchner, O. & Arnold, F.H. Strategies for the in vitro evolution of protein function: enzyme evolution by random recombination of improved sequences. J. Mol. Biol. 273, 336–347 (1997).
Wild, K., Bohner, T., Folkers, G. & Schulz, G.E. The structures of thymidine kinase from Herpes simplex virus type 1 in complex with substrates and a substrate analog. Protein Sci. 6, 2097–2106 (1997).
Brown, D.G. et al. 1995. Crystal structures of the thymidine kinase from herpes simplex virus type-1 in complex with deoxythymidine and ganciclovir. Nat. Struct. Biol. 2, 876– 881 (1995).
Champness, J.N. et al. Exploring the active site of herpes simplex virus type-1 thymidine kinase by x-ray crystallography of complexes with acyclovir and other ligands. Struct. Funct. Genet. 32, 350– 361 (1998).
Kussmann-Gerber, S., Kuonen, O., Folkers, G., Pilger, B.D. & Scapozza, L. Drug resistance of herpes simplex virus type 1: structural considerations on the molecular level of the thymidine kinase. Eur. J. Biochem. 255, 472–481 (1998).
Lavie, A. et al. Structure of thymidylate kinase reveals the cause behind the limiting step in AZT activation. Nat. Struct. Biol. 4, 601–604 (1997).
Lavie, A. et al. The bottleneck in AZT activation. Nat. Med. 3, 922–924 (1997).
Balzarini, J. et al. Improving AZT efficacy. Nat. Med. 4, 132 (1998).
Bouayadi, K. et al. Overexpression of DNA polymerase b sensitizes mammalian cells to 2´,3´-didexoycytidine and 3´-azidó-3´-deoxythymidine. Cancer Res. 57, 110–116 ( 1997).
Patten, P.A., Howard, R.J. & Stemmer, W.P.C. Applications of DNA shuffling to pharmaceuticals and vaccines. Curr. Opin. Biotechnol. 8, 724–733 (1997).
Igarashi, K., Hiraga, S. & Yura, T. A deoxythymidine kinase deficient mutant of Escherichia coli. II. Mapping and transduction studies with phage phi 80. Genetics 57, 643–654 ( 1967).
Cleland, W.W. Statistical analysis of enzyme data. Methods Enzymol. 63, 103–138 (1979).
Xu, Y. et al. X-ray analysis of azido-thymidine diphosphate binding to nucleoside diphosphate kinase. Proc. Natl. Acad. Sci. USA 94, 7162 –7165 (1997).
Perlman, D.A. et al. AMBER, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comp. Phys. Commun. 91, 1–41 ( 1995).
Jorgensen, W.L., Chrasekhar, J., Madura, J.D., Impey, R.W. & Klein, M. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Laskowski, R.A., MacArthur, M.W., Moos, D.S. & Thornton, J.M.J. PROCHECK: a program to check the stereochemical quality of protein structures. Appl. Cryst. 26, 283–291 (1993).
Goodford, P.J. A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J. Med. Chem. 28, 849–857 (1985).
Wade, R.C. & Goodford, P.J. Further development of hydrogen bond functions for use in determining energetically favorable binding sites on molecules of known structure. J. Med. Chem. 36, 140–156 (1993).
Acknowledgements
Thanks to Sun-Ai Raillard, Glenn Dawes, and Steve delCardayre for technical assistance, to the Computational Center of the ETH Zürich for computer time, and to Claus Krebber, Larry Loeb, Frances Arnold, and Rolf Zinkernagel for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christians, F., Scapozza, L., Crameri, A. et al. Directed evolution of thymidine kinase for AZT phosphorylation using DNA family shuffling. Nat Biotechnol 17, 259–264 (1999). https://doi.org/10.1038/7003
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/7003
This article is cited by
-
Algorithms for optimizing cross-overs in DNA shuffling
BMC Bioinformatics (2012)
-
Revealing biases inherent in recombination protocols
BMC Biotechnology (2007)
-
Direct positive selection for improved nitroreductase variants using SOS triggering of bacteriophage lambda lytic cycle
Gene Therapy (2007)
-
Drosophila deoxyribonucleoside kinase mutants with enhanced ability to phosphorylate purine analogs
Gene Therapy (2007)
-
Monitoring adenoviral DNA delivery, using a mutant herpes simplex virus type 1 thymidine kinase gene as a PET reporter gene
Gene Therapy (2002)