Abstract
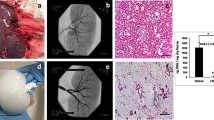
Avoidance of ice formation during cooling can be achieved by vitrification, which is defined as solidification in an amorphous glassy state that obviates ice nucleation and growth. We show that a vitrification approach to storing vascular tissue results in markedly improved tissue function compared with a standard method involving freezing. The maximum contractions achieved in vitrified vessels were >80% of fresh matched controls with similar drug sensitivities, whereas frozen vessels exhibited maximal contractions below 30% of controls and concomitant decreases in drug sensitivity. In vivo studies of vitrified vessel segments in an autologous transplant model showed no adverse effects of vitreous cryopreservation compared with fresh tissue grafts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Taylor, M.J. in Transplantation immunology: clinical and experimental (ed. Calne, R.Y.) 360–390 (Oxford University Press, New York, NY; 1984).
Pegg, D.E., Jacobsen, I.A., Armitage, W.J. & Taylor, M.J. in Organ preservation II (eds Pegg, D.E. & Jacobsen, I.A.) 132–146 (Churchill Livingstone, Edinburgh, UK; 1979).
Pegg, D.E. in Progress in transplantation (eds Morris, P.J. & Tilney, N.L.) 69–105 (Churchill Livingston, Edinburgh, UK; 1985).
Pegg, D.E. in The biophysics of organ preservation (eds Pegg, D.E. & Karow, A.M.) 117–140 (Plenum, New York, NY; 1987).
Hunt, C.J., Taylor, M.J. & Pegg, D.E. Freeze-substitution and isothermal freeze fixation studies to elucidate the pattern of ice formation on smooth muscle at 252 K (−21 C). J. Microsc. 125, 177–186 (1982).
Taylor, M.J. & Pegg, D.E. The effect of ice formation on the function of smooth muscle tissue following storage at −21 C and −60 C. Cryobiology 20, 36–40 (1982).
Jacobsen, I.A. et al. Effect of cooling rate and warming rate on glycerolized rabbit kidneys. Cryobiology 21, 637–653 (1984).
Fahy, G.M. in Biophysics of organ cryopreservation (eds Pegg, D.E. & Karow, A.M.) 265–297 (Plenum, New York, NY; 1987).
Fahy, G.M. in Low temperature biotechnology: emerging applications and engineering contributions (eds McGrath, J.J. & Diller, K.R.) 113–146 (The American Society of Mechanical Engineers, New York, NY; 1988).
Fahy, G.M., MacFarlane, D.R., Angell, C.A. & Meryman, H.T. Vitrification as an approach to cryopreservation. Cryobiology 21, 407–426 (1984).
Fahy, G.M., Levy, D. & Ali, S.E. Some emerging principles underlying the physical properties, biological actions, and utility of vitrification solutions. Cryobiology 24, 196–213 (1987).
Farrant, J. Mechanism of cell damage during freezing and thawing and its prevention. Nature 205, 1284–1287 (1965).
Elford, B.C. Diffusion and distribution of dimethyl sulphoxide in the isolated guinea-pig Taenia coli. J. Physiol. 209, 187–208 (1970).
Elford, B.C. & Walter, C.A. Preservation of structure and function of smooth muscle cooled to −79°C in unfrozen aqueous media. Nat. New Biol. 236, 58–60 (1972).
Elford, B.C. & Walter, C.A. Effects of electrolyte composition and pH on the structure and function of smooth muscle cooled to −79°C in unfrozen media. Cryobiology, 9, 82–100 (1972).
Kent, K.C., Whittemore, A.D. & Mannick, J.A. Short term and midterm results of an all-autogenous tissue policy for infrainguinal reconstruction. J. Vasc. Surg. 9, 107–114 (1989).
McNally, R.T., Walsh, K. & Richardson, W. Early clinical evaluation of cryopreserved allograft vein. Cryobiology 29, 702. (1992).
Walker, P.J., Mitchell, R.S., McFadden, P.M., James, D.R. & Mehigan, J.T. Early experience with cryopreserved saphenous vein allografts as a conduit for complex limb-salvage procedures. J. Vasc. Surg. 18, 561–569 (1993).
Bilfinger, T.V., Hartman, A.R., Liu, Y., Magazine, H.I. & Stefano, G.B. Cryopreserved veins in myocardial revascularization: possible mechanism for their increased failure. Ann. Thorac. Surg. 63, 1063–1069 (1997).
Brockbank, K.G.M. Effects of cryopreservation upon vein function in vivo. Cryobiology 31, 71–81 (1994).
Song, Y.C., Hunt, C.J. & Pegg, D.E. Cryopreservation of the common carotid artery of the rabbit. Cryobiology 31, 317–329 (1994).
Song, Y.C., Pegg, D.E. & Hunt, C.J. Cryopreservation of the common carotid artery of the rabbit: optimization of dimethyl sulfoxide concentration and cooling rate. Cryobiology 32, 405–421 (1995).
McNally, R.T., McCaa, C., Brockbank, K.G.M., Heacox, A.E. & Bank, H.L. Method for cryopreserving blood vessels. CryoLife, Inc. and Medical University of South Carolina. US Patent 5,145,769 (1992).
Finney, D.J. in Statistical methods in biological assay (ed. Finney, D.J.) 349–369 (Charles Griffin, London, 1978).
Faggioli, G.I. et al. Long term cryopreservation of autologous veins in rabbits. Cardiovasc. Surg. 2, 259–265 (1994).
Elmore, J.R., Gloviczki, P., Brockbank, K.G.M. & Miller, V.M. Cryopreservation affects endothelial and smooth muscle function of canine autogenous saphenous vein grafts. J. Vasc. Surg. 13, 584–592 (1991).
Rall, W.F. & Fahy, G. Ice-free cryopreservation of mouse embryos at −196°C by vitrification. Nature 313, 573–575 (1985).
Mehl, P. Nucleation and crystal growth in a vitrification solution tested for organ cryopreservation by vitrification. Cryobiology 30, 509–518 (1993).
Bateson, E.A.J., Busza, A.L., Pegg, D.E. & Taylor, M.J. Permeation of common carotid arteries with dimethyl sulfoxide. Cryobiology 31, 393–397 (1994).
Davies, M.G. et al. Controlling transplant vasculopathy in cryopreserved vein grafts with polyethylene glycol and glutathione during transport. Eur. J. Vasc. Endovasc. Surg. 17, 493–500 (1999)
Motulsky, H. Intuitive biostatistics (Oxford University Press, New York, NY; 1995).
Acknowledgements
Excellent technical assistance was provided by Janet Boggs, Beth Greene, and Kim McCourry. We thank Otto Hagen for advice relating to the physiological aspects of this study. This work was supported in part by a cooperative agreement (#97-07-0039) with the US Department of Commerce, National Institute of Standards Technology–Advanced Technology Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Y., Khirabadi, B., Lightfoot, F. et al. Vitreous cryopreservation maintains the function of vascular grafts. Nat Biotechnol 18, 296–299 (2000). https://doi.org/10.1038/73737
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/73737
This article is cited by
-
Pancreatic islet cryopreservation by vitrification achieves high viability, function, recovery and clinical scalability for transplantation
Nature Medicine (2022)
-
Rapid joule heating improves vitrification based cryopreservation
Nature Communications (2022)
-
It’s the Biology Orthopods! Heralding a Reconstructive Revolution Through Musculoskeletal Tissue Banks (MSTB) in India
Indian Journal of Orthopaedics (2022)
-
Liver Cryopreservation for Regenerative Medicine Applications
Regenerative Engineering and Translational Medicine (2021)
-
Different storage times and their effect on the bending load to failure testing of murine bone tissue
Scientific Reports (2020)