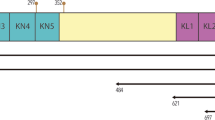
Abstract
Protease nexin I (PNI) is a potent anti–thrombin and anti–urokinase secreted by fibroblasts, and certain other extravascular cells. We describe the isolation, sequence, and expression of PNI cDNA cloned from a human foreskin fibroblast cDNA library. Two forms of PNI cDNA (designated α and β) have been identified. These differ only by the insertion of three nucleotides into the coding sequence, and apparently arise by utilization of an alternative splice acceptor site in the PNI gene. In the resulting proteins, αPNI contains an arg residue at position 310 where βPNI contains thr–gly residues. Both forms have been expressed in mammalian cells and inhibit thrombin and urokinase. The amino acid sequence of βPNI is identical to that of a recently described glial–derived neurite promoting factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Baker, J.B., Low, D.A., Simmer, R.L., and Cunningham, D.D. 1980. Protease nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell 21:37–45.
Baker, J.B., Knauer, D.J., and Cunningham, D.D. 1986. Protease nexins: secreted protease inhibitors that regulate protease actions at and near the cell surface, 153–172. In: The Receptors, Vol. 3. P. M. Conn, (ed.) Academic Press, NY.
Low, D.A., Scott, R.W., Baker, J.B., and Cunningham, D.D. 1982. Cells regulate their mitogenic response to thrombin through secretion of protease nexin. Nature 298:476–478.
Eaton, D.L., Scott, R.W., and Baker, J.B. 1984. Purification of human fibroblast urokinase proenzyme and analysis of its regulation by protease and protease nexin. J. Biol. Chem. 259:6241–6247.
Bergman, B.L., Scott, R.W., Bajpai, A., Watts, S., and Baker, J.B. 1986. Protease nexin I inhibits destruction of extracellular matrix by human tumor cells. Proc. Natl. Acad. Sci. USA 83:996–1000.
Gronke, R.S., Bergman, B.L., and Baker, J.B. 1987. Thrombin interaction with platelets: Influence of a platelet protease nexin. J. Biol. Chem. 262:3030–3036.
Carrell, R.W., and Boswell, D.R. 1987. Serpins: The super family of serins proteinase inhibitors, p.403–418. In: Proteinase Inhibitors. A. Barret and G. Salvesen (eds.) Elsevier, Amsterdam.
Rosenberg, R.D., and Damus, D.S. 1973. The purification and mechanism of action of human antithrombin-heparin cofactor. J. Biol. Chem. 248:6490–6505.
Scott, R.W., Bergman, B.L., Bajpai, A., Hersh, R.T., Rodriquez, H., Jones, B.N., Watts, S., and Baker, J.B. 1985. Protease nexin. Properties and a modified purification procedure. J. Biol. Chem. 260:7029–7034.
Gloor, S., Odink, K., Guenther, J., Nick, H., and Monard, D. 1986. A glia-derived neurite promoting factor with protease inhibitory activity belongs to the protease nexins. Cell 47:687–693.
Thomas, P. 1980. Hybridization of denatured RNA and small DNA fragments to nitrocellulose. Proc. Nal. Acad. Sci. USA 77:5201–5205.
Flamm, W., Bond, H., and Burr, H. 1966. Density gradient centrifuation of DNA in a fixed-angle rotor. Biochim. Biophys. Acta. 129:310–319.
Gubler, U. and Hoffman, B.J. 1983. A simple and very efficient method for generating cDNA libraries. Gene 25:263–269.
Young, R.A. and Davis, R.W. 1983. Efficient isolation of genes using antibody probes. Proc. Natl. Acad. Sci., U.S.A. 80:1194–1198.
Hunyh, T.V., Young, R.A., and Davis, R.W. In: DNA Cloning: A Practical Approach. Vol. 1. D. M. Glover (ed.) IRL Press.
Grunstein, M. and Hogness, D. 1975. Colony hybridization: A method for the isolation of cloned DNAs that contain a specific gene. Proc. Natl. Acad. Sci. U.S.A. 72:3961–3965.
Davis, R.W., Botstein, D., and Roth, J.R. 1980. Advanced Bacterial Genetics. Cold Springs Harbor Laboratory Press. Cold Spring Harbor, New York.
Rosenberg, S.A., Grimm, E.A., McGrogan, M., Doyle, M.E., Kawasaki, E., Koths, K., and Mark, D. 1984. Biological activity of recombinant human interleukin-2 produced in E. coli. Science. 223:1412–1414.
Messing, J. 1983. New M13 vectors for cloning. Methods Enzymol. 101:20–78.
Messing, J. and Viera, J. 1982. A new pair of MIS vectors for selecting either DNA strand of double-digest restriction fragments. Gene 19:269–276.
Sanger, F., Nicklen, S., and Coulson, R. 1977. DNA sequencing with chain termination inhibitors. Proc. Natl. Acad. Sci. U.S.A. 74:5463–5467.
Maniatis, T., Fritsch, E.F., and Sambrook, J. 1982. Molecular Cloning. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, New York.
Southern, E. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503–517.
Leff, S.E., Rosenfeld, M.G., and Evans, R.M. 1986. Complex transcriptional units: Diversity in gene expression by alternative DNA processing. Ann. Rev. Biochem. 55:1091–1117.
Breathnach, R. and Chambon, P. 1981. Organization and expression of eukaryotic split genes coding for proteins. Ann. Rev. Biochem. 50:349–383.
Padget, R.A., Grabowski, P.J., Konaska, M.M., Seiler, S., and Sharp, P.A. 1986. Splicing of messenger RNA precursors. Ann. Rev. Biochem. 55:1119–1150.
Gasser, G.S., Simonsen, C.C., Schilling, J.W., and Schimke, R.T. 1982. Expression of abbreviated mouse dihydrofolate reductase genes in cultured hamster cells. Proc. Natl. Acad. Sci. U.S.A. 79:6522–6528.
Kaufman, R.J. and Sharp, P.A. 1982. Construction of a modular dihydrofolate reductase cDNA gene: Analysis of signals utilized for efficient expression. Mol. Cell. Biol. 2:1304–1319.
Simonsen, C.C., and Levinson, A.D. 1983. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc. Natl. Acad. Sci. U.S.A. 80:2495–2499.
Simonsen, C.C., and Levinson, A.D. 1983. Analysis of processing and polyadenylation signals of the hepatitis B virus surface antigen using SV40-HBV chimeric plasmids. Molec. and Cell. Biol. 3:2250–2258.
Urlaub, G. and Chasin, L. 1980. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc. Natl. Acad. Sci. U.S.A. 77:4216–4220.
Monard, D., Nidey, E., Limat, A., Solomon, F. 1983. Inhibition of protease activity can lead to neurite extension in neuroblastoma cells. Prog. Brain Res. 58:359–364.
Ragg, H. 1986. A new member of the plasma protease gene family. Nucleic Acids Res. 14:1073–1084.
Hill, R.E., Shaw, P.H., Boyd, P.A., Baumann, H., and Hastie, N.D. 1984. Plasma protease inhibitors in mouse and human. Divergence within the reactive center regions. Nature 311:175–177.
Bock, S.C., Skriver, S., Nielsen, E., Thorgersen, H.-C., Wiman, B., Donaldson, V.H., Eddy, R.L., Marrinan, J., Radziejewska, E., Huber, R., Shows, T.B., and Magnusson, S. 1986. Human C1 inhibitor: Primary structure, cDNA cloning and chromosomal localization. Biochem. 25:4292–4301.
Carrell, R.W., Jeppson, J.-O., Laurell, C.-B., Brennan, S.O., Owen, M.C., Vaughan, L., and Boswell, D.R. 1982. Structure and variation of human α-1-antitrypsin. Nature 298:329–334.
Petersen, T.E., Dudek-Wojciechowska, G., Sottrup-Jensen, L., and Magnusson, S. 1979. Primary structure of antithrombin III (heparin cofactor): partial homology between α-1-antitrypsin and antithrombin III, p. 43–54. In: The Physiological Inhibitors of Coagulation and Fibrinolysis. Collen, D., Wiman, B., and Verstraete, M. (eds). Elsevier-North Holland Biomedical Press, Amsterdam.
Berger, S.L., and Berkenmeier, C.S. 1979. Inhibition of intractable nucleases with ribonucleoside-vanadyl complexes: Isolation of messenger ribonucleic acid from resting lymphocytes. Biochem. 18:5143–5149.
Aviv, J., and Leder, P. 1972. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc. Natl. Acad. Sci. U.S.A. 69:1408–1412.
Frischauf, A., Lehrach, H., Poustka, A., and Murray, N. 1983. Lambda replacement vectors carrying polylinker sequences. J. Mol. Biol. 170:827–842.
Rigby, P.W.J., Dieckman, M., Rhodes, C., and Berg, P. 1977. Labeling deonyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J. Mol. Biol. 113:237–251.
Crouse, G.F., Simonsen, C.C., McEwan, R.N., and Schimke, R.T. 1982. Structure of amplified normal and variant dihydrofolate reductase genes in mouse sarcoma S180 cells. J. Biol. Chem. 257:7887–7897.
Kaplan, G., Unkeless, J.C., and Cohn, Z.A. 1979. Insertion and turnover of macrophage plasma membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 76:3824–3828.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
Baker, J.B., Low, D.A., Eaton, D.L., and Cunningham, D.D. 1982. Thrombin-mediated mitogenesis: The role of secreted protease nexin. J. Cell. Physiol. 112:291–297.
Sommer, J., Gloor, S., Rovelli, G.F., Hofsteenge, J., Nick, H., and Meier, R., and Monard, D. 1987. cDNA sequence coding for a rat gliaderived nexin and its homology to members of the serpin family. Biochem. 26:6407–6410.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McGrogan, M., Kennedy, J., Ping Li, M. et al. Molecular Cloning and Expression of Two Forms of Human Protease Nexin I. Nat Biotechnol 6, 172–177 (1988). https://doi.org/10.1038/nbt0288-172
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0288-172