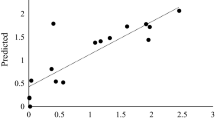
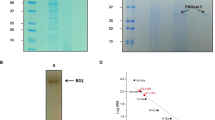
Abstract
The extremely thermostable wild type and recombinant β-glucosidases, from Pyrococcus furiosus, served as catalysts for the biotransformation of new glucoconjugates at elevated temperatures. In conversion experiments using the transglucosylation approach, the free or immobilized enzyme accepted primary and even tertiary organic alcohols, as well as primary and secondary artificial organosilicon alcohols, as aglycones. Cellobiose served as the glucose donor. The products obtained were purified by liquid chromatography and analyzed. Using β-glucosidase a wide variety of products were synthesized. Due to the very broad structural diversity of the aglycones linked to the 1-β-O-D-glucose, this β-glucosidase seems to be a useful biocatalyst for regio- and stereoselective sugar derivative synthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Adams, M. W. W., Perler, F. B. and Kelly, R. M. 1995. Extremozymes: expanding the limits of biocatalysis. Bio/Technology 13: 662–668.
Hodgson, J. 1995. New formulas for carbohydrates. Bio/Technology 13: 38–41.
Nilsson, K. G. I. 1991. Enzymatic synthesis of complex carbohydrates and their glycosides, p. 117–178. In: Applied Biocatalysis, Vol. 1. Blanch, H. W. and Clark, D. S. (Eds.). Dekker Inc., New York.
Kengen, S. W. M., Luesink, E. J., Stams, A. J. M. and Zehnder, A. J. B. 1993. Purification and characterization of an extremely thermostable β-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur. J. Biochem. 213: 305–312.
Fiala, G. and Stetter, K. O. 1986. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 145: 56–61.
Woese, C.R., Kandler, O. and Wheelis, M. L. 1990. Towards a natural system of organisms: Proposal for the domains Archae, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 87: 4576–4579.
Kengen, S. W. M. and Stams, A. J. M. 1994. An extremely thermostable (β-glucosidase from the thermophilic archaeon Pyrococcus furiosus; a comparison with other glycosidases. Biocatalysis 11: 79–88.
Voorhorst, W. G. B., Eggen, R. I. L., Luesink, E. J. and d de Vos, W. M. 1995. Characterization of the celbB gene coding for the β-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus and its expression and site-directed Mutation in Escherischia coli. J. Bacteriol. 177(24). In press.
Gunata, Z., Vallier, M. J., Sapis, J. C., Baumes, R. and Bayonove, C. 1994. Enzymatic synthesis of monoterpenyl (β-D-glucosides by various β-glucosidases. Enzyme Microbial Technology 16: 1055–1058.
Vic, G., Biton, J., Le Beller, D., Michel, J. -M. and Thomas, D. 1995. Enzymatic glucosylation of hydrophobic alcohols in organic medium by the reverse hydrolysis reaction using almond-β-D-glucosidase. Biotechnol. Bioengin. 46: 109–116.
Vulfson, E. N., Patel, R., Beecher, J. E., Andrews, A. T. and Law, B. A. 1990. Glycosidases in organic solvents: I. Alkyl-β-glucoside synthesis in a water-organic two-phase system. Enzyme Microbial Technology 12: 950–954.
Laroute, V. and Willemot, R. -M. 1992. Glucoside synthesis by glucoamylase or β-glucosidase in organic solvents. Biotechnology Letters 14: 169–174.
Ravet, C., Thomas, D. and Legoy, M. D. 1993. Gluco-oligosaccharide synthesis by free and immobilized β-glucosidase. Biotech. and Bioeng. 42: 303–308.
Tacke, R. and Linoh, H. 1989. Bioorganosilicon chemistry, p. 1143–1206. In: The Chemistry of Organic Silicon Compounds, Part 2. Patai, S. and Rappoport, Z. (Eds.). Wiley, Chichester.
Fischer, L. 1995. Capacity of the yeast Trigonopsis variabilis (DSM 70714) for the enantioselective reduction of organosilikon compounds. Adv. in Mol. and Cell Biol. In press.
Trincone, A., Improta, R., Nucci, R., Rossi, M. and Gambacorta, A. 1994. Enzymatic synthesis of carbohydrate derivatives using β-glucosidase of Sulfolobus solftaricus. Biocatalysis 10: 195–210.
Trincone, A. and Pagnotta, E. 1995. Efficient chemoselective synthesis of 3,4′,dihydroxypropiophenone 3-O-β-D-glucoside by thermophilic β-glycosi-dase from Sulfolobus solfactaricus. Biotechnol. Lett. 17: 45–48.
Fischer, L., Bromann, R. and Wagner, F. 1995. Enantioselective synthesis of several 1-O-β-D-glucoconjugates using almond β-glucosidase. Biotechnol. Lett. 17(11):1169–1174.
Kezdy, F. and Bender, M. 1962. The kinetics of the α-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochem. 1: 1097–1104.
Organikum . 1988. VEB Deutscher Verlag der Wissenschaften, Berlin: 411.
Tacke, R., Wagner, S. A., Brakmann, S., Wuttke, F., Eilert, U., Fischer, L. and Syldatk, C. 1993. Synthesis of acetyldimethyl(phenyl)silane and its enantioselective conversion into (R)-(1-hydroxyethyl)dimethyl(phenyl)silane by plant cell suspension cultures of Symphytum officinale L. and Ruta graveolens L. J. Organomet. Chem. 458: 13–17.
Syldatk, C., Andree, H., Stoffregen, A., Wagner, F., Stumpf, B., Ernst, L., Zilch, H. and Tacke, R. 1987. Enantioselective reduction of acetyl-dimethylphenylsilane by Trigonopsis variabilis (DSM 70714)., Appl. Microbiol. Biotechnol. 27: 152–158.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, L., Bromann, R., Kengen, S. et al. Catalytical Potency of β-Glucosidase from the Extremophile Pyrococcus furiosus in Glucoconjugate Synthesis. Nat Biotechnol 14, 88–91 (1996). https://doi.org/10.1038/nbt0196-88
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0196-88
This article is cited by
-
Enzymatic production of lactulose and 1-lactulose: current state and perspectives
Applied Microbiology and Biotechnology (2013)
-
Recombinant production of hyperthermostable CelB from Pyrococcus furiosus in Lactobacillus sp.
Applied Microbiology and Biotechnology (2012)