Abstract
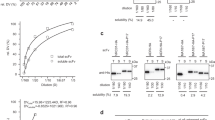
The Fv and Fab fragment and both orientations of the single-chain Fv fragment (VH-linker-VL and VL-linker-VH) of an antibody can be expressed in functional form in the periplasm of Escherichia coli, but the yield of these correctly assembled proteins is limited by the periplasmic folding process. While the periplasmic E. coli disulfide isomerase DsbA is required for this assembly, its functional over-expression does not significantly change the folding limit. Similarly, the functionally over-expressed E. coli proline cis-trans isomerase does not change the amount of all but one of the antibody fragments, not even if DsbA is over-expressed as well. Therefore, aggregation steps in the periplasm appear to compete with periplasmic folding, and they may occur before disulfide formation and/or proline cis-trans isomerization takes place and be independent of their extent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Skerra, A. and Plückthun, A. 1988. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science 240: 1038–1041.
Better, M., Chang, C.P., Robinson, R.R. and Horwitz, A.H. 1988. Escherichia coli secretion of an active chimeric antibody fragment. Science 240: 1041–1043.
Glockshuber, R., Malia, M., Pfitzinger, I. and Plückthun, A. 1990. A comparison to stabilize immunoglobulin Fv-fragments. Biochemistry 29: 1362–1367.
Skerra, A. and Plückthun, A. 1991. Secretion and in vivo folding of the Fab fragment of the antibody McPC603 in Escherichia coli—influence of disulfides and cis-prolines. Protein Eng. 4: 971–979.
Anfinsen, C.B. 1973. Principles that govern the folding of protein chains. Science 181: 223–230.
Creighton, T.E. 1978. Experimental studies of protein folding and unfolding. Prog. Biophys. Mol. Biol. 33: 231–297.
Jaenicke, R. 1987. Folding and association of proteins. Prog. Biophys. Mol. Biol. 49: 117–237.
Fischer, G. and Schmid, F.X. 1990. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry 29: 2205–2212.
Lorimer, G.H. 1992. Role of accessory proteins in protein folding. Curr. Opin. Struct. Biol. 2: 26–34.
Jaenicke, R. 1991. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry 30: 3147–3161.
Gething, M.J. and Sambrook, J. 1992. Protein folding in the cell. Nature 355: 33–45.
Kiefhaber, T., Rudolph, R., Kohler, H.H. and Buchner, J. 1991. Protein aggregation in vitro and in vivo: a quantitative model of the kinetic competition between folding and aggregation. Bio/Technology 9: 825–829.
Fischer, G., Bang, H., Mech, C. 1984. Nachweis einer Enzymkatalyse für die cis-trans-Isomerisierung der Peptidbindung in prolinhaltigen Peptiden. Biomed. Biochem. Acta 43: 1101–1111.
Brandts, J.F., Halvorson, H.R. and Brennan, M. 1975. Consideration of the possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry 14: 4953–4963.
Schmid, F.X. and Baldwin, R.L. 1978. Acid catalysis of the formation of the slow-folding species of RNase A: evidence that the reaction is proline isomerization. Proc. Natl. Acad. Sci. USA 75: 4764–4768.
Schmid, F.X., Grafl, R., Wrba, A. and Beintema, J.J. 1986. Role of proline peptide bond isomerization in unfolding and refolding of ribonuclease. Proc. Natl. Acad. Sci. USA 83: 872–876.
Grathwohl, C. and Wüthrich, K. 1976. NMR studies of the molecular conformations in the linear oligopeptides H-(I-Ala)n-I-Pro-OH. Biopolymers 15: 2043–2057.
Juy, M., Lam-Thanh, H., Lintner, K. and Fermandjian, S. 1983. Conformation and mobility of tyrosine side chain in tetrapeptides. Int. J. Pept. Prot. Res. 22: 437–449.
Stewart, D.E., Sarkar, A. and Wampler, J.E. 1990. Occurence and role of cis peptide bonds in protein structures. J. Mol. Biol. 214: 253–260.
Kiefhaber, T., Quaas, R., Hahn, U. and Schmid, F.X. 1990. Folding of ribonuclease T1. 1. Existence of multiple unfolded states created by proline isomerization. Biochemistry 29: 3053–3061.
Fransson, C., Freskgard, P.O., Herbertsson, H., Johansson, A., Jonasson, P., Martensson, L.G., Svensson, M., Jonsson, B.H. and Carlsson, U. 1992. Cis-trans isomerization is rate-determining in the reactivation of denatured human carbonic anhydrase-II as evidenced by proline isomerase. FEBS Letters 296: 90–94.
Lang, K., Schmid, F.X. and Fischer, G. 1987. Catalysis of protein folding by prolyl isomerase. Nature 329: 268–270.
Schönbrunner, E.R., Mayer, S., Tropschug, M., Fischer, G., Takahashi, N. and Schmid, F.X. 1991. Catalysis of protein folding by cyclophilins from different species. J. Biol. Chem. 266: 3630–3635.
Lin, L.-N., Hasumi, H. and Brandts, J.F. 1988. Catalysis of proline isomerization during protein-folding reactions. Biochim. Biophys. Acta 956: 256–266.
Goto, Y. and Hamaguchi, K. 1982. Unfolding and refolding of the constant fragment of the immunoglobulin light chain. J. Mol. Biol. 156: 891–910.
Buchner, J., Brinkmann, U. and Pastan, I. 1992. Renaturation of a single-chain immunotoxin facilitated by chaperones and protein disulfide isomerase. Bio/Technology 10: 682–685.
Liu, J. and Walsh, C.T. 1990. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmatic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc. Natl. Acad. Sci. USA 87: 4028–4032.
Hayano, T., Takahashi, N., Kato, S., Maki, N. and Suzuki, M. 1991. Two distinct forms of peptidylprolyl-cis-trans-isomerase are expressed separately in periplasmic and cytoplasmic compartments of Escherichia coli cells. Biochemistry 30: 3041–3048.
Harrison, R.K. and Stein, R.L. 1990. Substrate specificities of the peptidyl prolyl cis-trans isomerase activities of cyclophilin and FK-506 binding protein: evidence for the existence of a family of distinct enzymes. Biochemistry 29: 3813–3816.
Venetianer, P. and Straub, F.B. 1963. The enzymic reactivation of reduced ribonuclease. Biochim. Biophys. Acta 67: 166–168.
Goldberger, R.F., Epstein, C.J. and Anfinsen, C.B. 1963. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J. Biol. Chem. 238: 628–635.
Freedman, R.B., Bulleid, N.J., Hawkins, H.C. and Paver, J.L. 1989. Role of protein disulphide-isomerase in the expression of native proteins. Biochem. Soc. Symp. 55: 167–192.
Parkkonen, T., Kivirikko, K.I. and Pihlajaniemi, T. 1988. Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase β-subunit, protein disulphide-isomerase and a cellular thyroid-hormone binding protein. Biochem. J. 256: 1005–1011.
Gilbert, H.F. 1990. Molecular and cellular aspects of thiol-disulfide exchange. Adv. Enzymol. 63: 69–172.
Bardwell, J.C.A., McGovern, K. and Beckwith, J. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67: 581–589.
Kamitani, S., Akiyama, Y. and Ito, K. 1992. Identification and characterization of an Escherichia coli gene required for the formation of correctly folded alkaline-phosphatase, a periplasmic enzyme. EMBO J. 11: 57–62.
Yu, J., Webb, H. and Hirst, T.R. 1992. A homologue of the Escherichia coli DsbA protein involved in disulphide bond formation is required for enterotoxin biogenesis in Vibrio cholerae. Mol. Microbiol. 6: 1949–1958.
Satow, Y., Cohen, G.H., Padlan, E.A. and Davies, D.R. 1986. Phosphocholine binding immunoglobulin Fab McPC603. J. Mol. Biol. 190: 593–604.
Skerra, A., Pfitzinger, I. and Plückthun, A. 1991. The functional expression of antibody Fv fragments in Escherichia coli: improved vectors and a generally applicable purification technique. Bio/Technology 9: 273–278.
Anand, N.N., Mandal, S., MacKenzie, C.R., Sadowska, J., Sigurskjold, B., Young, N.M., Bundle, D.R. and Narang, S.A. 1991. Bacterial expression and secretion of various single-chain Fv genes encoding proteins specific for a Salmonella serotype B O-antigen. J. Biol. Chem. 266: 21874–21879.
Pantoliano, M.W., Bird, R.E., Johnson, S., Asel, E.D., Dodd, S.W., Wood, J.F. and Hardman, K.D. 1991. Conformational stability, folding, and ligand-binding affinity of single-chain-Fv immunoglobulin fragments expressed in Escherichia coli. Biochemistry 30: 10117–10125.
Pace, C.N. 1990. Measuring and increasing protein stability. Trends Biotechnol. 8: 93–98.
Kunkel, T.A., Roberts, J.D. and Zakour, R.A. 1987. Rapid and efficient site specific mutagenesis without phenotypic selection. Methods Enzymol. 154: 367–382.
O'Callaghan, C.H., Morris, A., Kirby, S.M. and Shingler, A.H. 1972. Novel method for detection of β-lactamases by using chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1: 283–288.
Holmgren, A. 1979. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 254: 9627–9632.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Knappik, A., Krebber, C. & Plückthun, A. The Effect of Folding Catalysts on the In Vivo Folding Process of Different Antibody Fragments Expressed in Escherichia coli. Nat Biotechnol 11, 77–83 (1993). https://doi.org/10.1038/nbt0193-77
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0193-77
This article is cited by
-
Prokaryotic expression of antibodies
Cancer and Metastasis Reviews (2005)