Abstract
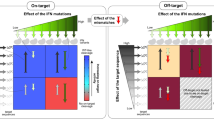
Despite the utility of CRISPR–Cas9 nucleases for genome editing, the potential for off-target activity limits their application, especially for therapeutic purposes1,2. We developed a yeast-based assay to identify optimized Streptococcus pyogenes Cas9 (SpCas9) variants that enables simultaneous evaluation of on- and off-target activity. We screened a library of SpCas9 variants carrying random mutations in the REC3 domain and identified mutations that increased editing accuracy while maintaining editing efficiency. We combined four beneficial mutations to generate evoCas9, a variant that has fidelity exceeding both wild-type (79-fold improvement) and rationally designed Cas9 variants3,4 (fourfold average improvement), while maintaining near wild-type on-target editing efficiency (90% median residual activity). Evaluating evoCas9 on endogenous genomic loci, we demonstrated a substantially improved specificity and observed no off-target sites for four of the eight single guide RNAs (sgRNAs) tested. Finally, we showed that following long-term expression (40 d), evoCas9 strongly limited the nonspecific cleavage of a difficult-to-discriminate off-target site and fully abrogated the cleavage of two additional off-target sites.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Primary accessions
BioProject
Protein Data Bank
Referenced accessions
Protein Data Bank
References
Sander, J.D. & Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
Nuñez, J.K., Harrington, L.B. & Doudna, J.A. Chemical and biophysical modulation of Cas9 for tunable genome engineering. ACS Chem. Biol. 11, 681–688 (2016).
Slaymaker, I.M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Kleinstiver, B.P. et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016).
Kim, S., Kim, D., Cho, S.W., Kim, J. & Kim, J.S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014).
Tsai, S.Q. et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 33, 187–197 (2015).
Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 24, 1020–1027 (2014).
Liang, X. et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 208, 44–53 (2015).
Yin, H. et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat. Biotechnol. 34, 328–333 (2016).
Petris, G. et al. Hit and go CAS9 delivered through a lentiviral based self-limiting circuit. Nat. Commun. 8, 15334 (2017).
Mali, P. et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838 (2013).
Ran, F.A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Shen, B. et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat. Methods 11, 399–402 (2014).
Guilinger, J.P., Thompson, D.B. & Liu, D.R. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat. Biotechnol. 32, 577–582 (2014).
Tsai, S.Q. et al. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat. Biotechnol. 32, 569–576 (2014).
Bolukbasi, M.F. et al. DNA-binding-domain fusions enhance the targeting range and precision of Cas9. Nat. Methods 12, 1150–1156 (2015).
Fu, Y., Sander, J.D., Reyon, D., Cascio, V.M. & Joung, J.K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284 (2014).
Kim, D., Kim, S., Kim, S., Park, J. & Kim, J.-S. Genome-wide target specificities of CRISPR-Cas9 nucleases revealed by multiplex Digenome-seq. Genome Res. 26, 406–415 (2016).
Chen, J.S. et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 550, 407–410 (2017).
Doyon, Y. et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 26, 702–708 (2008).
Zhang, F. et al. High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc. Natl. Acad. Sci. USA 107, 12028–12033 (2010).
Chen, B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013).
Brinkman, E.K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168 (2014).
Ran, F.A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Kim, H.K. et al. In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nat. Methods 14, 153–159 (2017).
Burstein, D. et al. New CRISPR-Cas systems from uncultivated microbes. Nature 542, 237–241 (2017).
Shmakov, S. et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 15, 169–182 (2017).
DiCarlo, J.E. et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41, 4336–4343 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Perez-Pinera, P. et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 10, 973–976 (2013).
Jegga, A.G., Inga, A., Menendez, D., Aronow, B.J. & Resnick, M.A. Functional evolution of the p53 regulatory network through its target response elements. Proc. Natl. Acad. Sci. USA 105, 944–949 (2008).
Tomso, D.J. et al. Functionally distinct polymorphic sequences in the human genome that are targets for p53 transactivation. Proc. Natl. Acad. Sci. USA 102, 6431–6436 (2005).
Gietz, R.D. & Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 31–34 (2007).
Stuckey, S. & Storici, F. Gene knockouts, in vivo site-directed mutagenesis and other modifications using the delitto perfetto system in Saccharomyces cerevisiae. Methods Enzymol. 533, 103–131 (2013).
Geissmann, Q. OpenCFU, a new free and open-source software to count cell colonies and other circular objects. PLoS One 8, e54072–e10 (2013).
Casini, A., Olivieri, M., Vecchi, L., Burrone, O.R. & Cereseto, A. Reduction of HIV-1 infectivity through endoplasmic reticulum-associated degradation-mediated Env depletion. J. Virol. 89, 2966–2971 (2015).
Tsai, S.Q., Topkar, V.V., Joung, J.K. & Aryee, M.J. Open-source guideseq software for analysis of GUIDE-seq data. Nat. Biotechnol. 34, 483 (2016).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
Kleinstiver, B.P. et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523, 481–485 (2015).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Acknowledgements
The authors wish to thank the LaBSSAH–CIBIO Next Generation Sequencing Facility of the University of Trento for sequencing samples. We are grateful to D. Arosio for helpful discussion throughout the development of this study. This work was supported by intramural funding from the University of Trento and by the European Research Council grant ERCCoG648670 (F.D.).
Author information
Authors and Affiliations
Contributions
A.Ca., M.O., C.M., G.R., and G.P. designed and performed the experiments; A.Ca., M.O., G.R., C.M., G.M. and G.P. collected and analyzed the data; F.L., D.P., A.R. and F.D. contributed with GUIDE-seq experiments and targeted deep-sequencing analysis; A.I. contributed with the yeast assay design and setup. A.Ca., M.O., G.P. and A.C. conceived and designed the study, wrote and edited the paper; A.C. was responsible for the coordination of the study. All authors read, corrected and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.C., A.Ca., G.P., A.I., and M.O. are the inventors of the filed patent on the high-fidelity SpCas9 variants.
Integrated supplementary information
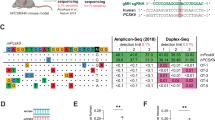
Supplementary Figure 1 Yeast screening for high-specificity SpCas9 variants
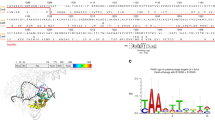
(a) Top panel: scheme of SpCas9 domains. The REC3 domain is part of the recognition lobe. BH: bridge helix. PI: PAM-interacting domain. Bottom panel: hybrid surface/ribbon structure (PDB ID: 4UN3) of SpCas9 in complex with sgRNA and target DNA. The REC3 alpha helical domain is highlighted in blue, while the rest of the structure is coloured in grey. RNA is represented in orange, DNA in red. (b) Distribution and frequency of amino acid substitutions obtained from the yeast screening. Green bars indicate substitutions belonging to mutants which showed more than 75% of residual on-target activity and more than 3-fold increase in specificity compared to wild-type SpCas9, while grey bars indicate substitutions included in variants failing to meet these requirements. Red bars indicate the subset mutations belonging to highly active and specific variants that are also in close proximity to the target DNA:gRNA duplex.
Supplementary Figure 2 Evaluation of R661 residue to improve on-target activity of the VNEL SpCas9 variant
(a) SpCas9 crystal structure (PDB ID: 4OO8) showing arginine 661 and its predicted interactions with the target DNA:guide RNA heteroduplex. While the R661L substitution likely introduces clash interactions (indicated by red disks) with the sgRNA backbone in all its rotamers (a representative rotamer is shown), the R661Q and R661S mutations are predicted to preserve one and two polar contacts with the guide, respectively, and simultaneously disrupt bonding interaction with the target DNA backbone. (b) 293multiEGFP were transfected with wild-type SpCas9, evoCas9 or the VNEL variant together with sgRNAs targeting different regions of the EGFP coding sequence. The loss of EGFP fluorescence (reported as mean percentage) was measured by FACS analysis 7 days post-transfection. (c) Editing of five genomic loci was evaluated in 293T cells after transfection with wild-type SpCas9, the VNEQ (evoCas9) variant or the VNEL variant together with the corresponding sgRNA. Indel formation (reported as mean percentage) was measured using the TIDE tool 7 days post-transfection. In panels (b,c) individual biologically independent samples are represented as overlaid circles.
Supplementary Figure 3 evoCas9 intracellular expression and titration of activity
(a) Representative western blot of lysates from 293T cells transfected with wild-type SpCas9, evoCas9 or the other high-fidelity variants, as indicated, together with the sgGFPon sgRNA. The graph below the blot reports the mean densitometric quantification of SpCas9 expression normalized on tubulin levels. Tubulin was used as a loading control. SpCas9 was detected using an anti-FLAG antibody. (b) Titration curve of evoCas9 activity in 293multiEGFP cells. Different amounts of evoCas9 or wild-type SpCas9 were transfected together with a fixed quantity of a sgRNA targeting the EGFP CDS (sgGFPon). Loss of EGFP fluorescence (reported as mean percentage) was measured by FACS at 7 days post-transfection. (c) Representative western blot of lysates from 293T cells transduced with lentiviral vectors expressing either wild-type SpCas9, evoCas9 or SpCas9-HF1, as indicated, and the sgGFPon sgRNA. The graph below the blot reports the mean densitometric quantification of SpCas9 expression normalized on actin levels. Actin was used as a loading control. SpCas9 was detected using an anti-FLAG antibody. Lysates were collected 20 days post-transduction and selection for stable SpCas9 expression. In all panels n=2 biologically independent samples (reported as overlaid circles).
Supplementary Figure 4 sgRNA requirements for evoCas9 activity
(a) The mean on-target cleavage activity of evoCas9, SpCas9-HF1 and eSpCas9(1.1) was compared to wild-type SpCas9 using truncated sgRNAs (17-19 nt) targeting EGFP; (b) a sgRNA targeting EGFP without a 5’-G (site20) and the same sgRNA containing a mismatched 5’-G nucleotide (site20+G). (c) evoCas9 editing activity in combination with sgRNAs targeting the EMX1-R and CXCR4 endogenous loci containing a 5’ mismatched guanine. (d) Evaluation of the effect of spacer length on evoCas9, SpCas9-HF1 and eSpCas9(1.1) editing activity. Mean EGFP knockdown was measured in the presence of sgRNAs with spacers spanning a length of 19-23 nt either containing a mismatched 5’-G or fully annealing with the target, as indicated. A schematic representation of the tested sgRNAs is reported in the panel above. Red squares indicate non-matching guanines; orange squares indicate extra matching nucleotides extending the spacer. (e) evoCas9, SpCas9-HF1 and eSpCas9(1.1) mean cleavage activity compared to wild-type SpCas9 in 293multiEGFP cells using sgRNAs characterized either by fully matching spacers starting with non-G nucleotides or with a mismatched 5’-G. (f) evoCas9 mean activity and specificity using optimized sgRNAs. evoCas9 together with wild-type SpCas9 and two previously published variants (SpCas9-HF1, eSpCas9(1.1)) were tested in an EGFP disruption assay using sgRNA with optimized scaffolds8 (extended stem-loop and base-flip) including the same spacers used in Fig. 2. For all the experiments loss of EGFP fluorescence was measured by FACS analysis at 7 days post transfection. For endogenous loci indel analysis cells were collected at 7 days post-transfection. Dashed lines indicate the background loss of EGFP fluorescence. Individual biologically independent samples are represented as overlaid circles.
Supplementary Figure 5 Evaluation of evoCas9 genome-wide specificity by GUIDE-seq (CCR5, CXCR4, FANCF2, HEK site4, EMX1, PD1)
GUIDE-seq profiles obtained with eight sgRNAs targeting the CCR5, CXCR4, FANCF2, HEK site4, EMX1, PD1 genomic loci in combination with wild-type SpCas9, SpCas9-HF1, eSpCas9(1.1) and evoCas9. GUIDE-seq read counts for the different SpCas9 are reported on the right of the sequences. (-) indicates that the off-target was not detected in the corresponding sample. Mismatched positions are indicated with colored boxes. Black squares indicate on-target sites. DNA from three biological replicates was mixed before library preparation. Additional information on the identified off-targets is reported in Supplementary Data 1, 2.
Supplementary Figure 6 Evaluation of evoCas9 genome-wide specificity by GUIDE-seq (VEGFA2, VEGFA3)
GUIDE-seq profiles obtained with eight sgRNAs targeting the VEGFA2 and VEGFA3 genomic loci in combination with wild-type SpCas9, SpCas9-HF1, eSpCas9(1.1) and evoCas9. GUIDE-seq read counts for the different SpCas9 are reported on the right of the sequences. (-) indicates that the off-target was not detected in the corresponding sample. For the VEGFA2 sgRNA the off-target list is limited to the sites with higher reads than the on-target obtained with wild-type SpCas9 and all the sites detected with evoCas9, SpCas9-HF1 and eSpCas9(1.1). Mismatched positions are indicated with colored boxes. Black squares indicate on-target sites. DNA from three biological replicates was mixed before library preparation. Additional information on the identified off-targets is reported in Supplementary Data 1, 2.
Supplementary Figure 7 GUIDE-seq on-target specificities of high-fidelity SpCas9 variants
Cleavage specificity, expressed by the percentage of on-target reads captured by GUIDE-seq (from Fig. 3a and Supplementary Fig. 5, 6) using wild-type SpCas9, SpCas9-HF1, eSpCas9(1.1) and evoCas9, in combination with sgRNAs targeting the VEGFA2, VEGFA3, EMX1, CCR5, CXCR4, FANCF2, PD1 and HEK site4 loci.
Supplementary Figure 8 Evaluation of evoCas9 specificity by targeted deep-sequencing
(a) On-/off-target ratios for the nine common off-targets associated to the VEGFA2 locus calculated from the data in Fig. 3g and reported for wild-type SpCas9 and each different high-fidelity variant, as indicated in the graph. The bars indicate the interval between the values of the ratios obtained from the two replicate samples. Indel analysis of previously validated off-target sites relative to the EMX1 locus (b) and the VEGFA3 locus (c) performed by targeted deep-sequencing on genomic DNA of 293T cells expressing wild-type SpCas9, SpCas9-HF1, eSpCas9(1.1) or evoCas9 together with each specific sgRNA. Cells not expressing SpCas9 were sequenced to determine background indel levels. Two independent experiments were performed by mixing genomic DNA from three biological replicates before library preparation. The shaded circles represent the two measured editing percentages, while the solid circle indicates the mean.
Supplementary Figure 9 Side-by-side comparison of evoCas9, SpCas9-HF1 and eSpCas9(1.1) specificity on selected therapeutically relevant genes
293T cells were transfected with wild-type SpCas9, SpCas9-HF1, eSpCas9(1.1) or evoCas9 together with sgRNAs targeting the FANCF2 (a) or the CCR5 loci (b). Mean indel formation at the on-targets and at two previously validated off-target sites (one for each locus) was evaluated 7 days post-transfection using the TIDE tool. The sequences of the on- and off-target sites for each locus are reported above the corresponding graphs; the red square indicates the mismatched base. (c) On/off ratios calculated from the mean indel percentages obtained in (a). The dotted lines indicate evoCas9 fold increase in specificity. (d) Schematic representation of the CCR5 locus and its off-target site in the highly homologous CCR2 gene. Simultaneous cleavage of the two sites generates a chromosomal deletion of approximately 16 kb. Semi-quantitative PCR was performed on genomic DNA of 293T cells transfected with wild-type SpCas9, SpCas9-HF1, eSpCas9(1.1) or evoCas9 and the CCR5 sgRNA to assess the amount of chromosomal deletion generated in each condition. The FANCF locus was used as an internal normalizer. The amount of deletion was quantified using densitometry with ImageJ. The numbers above the dotted line indicates evoCas9 fold increase in specificity relative to the connected histogram bars. For all panels n=2 biologically independent samples (represented as overlaid circles).
Supplementary Figure 10 Comparison of evoCas9 and HypaCas9 specificity by meta-analysis
Meta-analysis of GUIDE-seq experimental data relative to evoCas9 and HypaCas9 (from Chen et al., Nature 2017). (a) Total number of off-target sites detected by GUIDE-seq for the VEGFA2, VEGFA3 and FANCF2 sgRNAs in combination with evoCas9 and HypaCas9. (b) Radar plot reporting the global distribution of the detected off-target sites for evoCas9 and HypaCas9 according to their number of mismatches (1 to 5). (c) Venn diagram showing the intersection among all the off-targets identified with evoCas9 and HypaCas9. On-target cleavage specificity, measured as the percentage of GUIDE-seq reads captured by the on-target site for the corresponding locus, reported for each tested sgRNA (as indicated in the graphs, d) or for the whole dataset (e) for evoCas9 and HypaCas9. (f) On-/off-target ratios calculated from the GUIDE-seq reads obtained for the 9 off-target sites common to evoCas9 and HypaCas9. Statistical significance was assessed using the two-sided Wilcoxon rank sum test, sample size n=9. Summary of data distributions and statistical details are reported in Supplementary Table 5. ns, not significant.
Supplementary Figure 11 evo-dCas9 transcriptional activation
(a) Schematic representation of the Tet Responsive Element (TRE)-EGFP based transcriptional activator reporter. Upon binding of dCas9-VP64 to TetO repeats EGFP expression is activated. (b) EGFP activation was measured in 293T cells transfected with dCas9 or evo-dCas9 based transcriptional activators together with matching sgRNAs (both with or without a 5’ mismatched G) or mismatched sgRNA, as indicated. TetO-off6 contains a mismatch in position 6 from the PAM, TetO-off1314 contains two mismatches in positions 13-14 and TetO-off1819 contains two mismatches in positions 18-19. n=2 biologically independent samples are represented as overlaid circles. EGFP expression was measured by FACS analysis at 2 days post-transfection. (c) Fold activation of EGFP expression with respect to the non-targeting control calculated from the data in (b). Individual values are reported as overlaid circles.
Supplementary Figure 12 Reproducibility of wild-type SpCas9 GUIDE-seq experiments across independent studies
Scatter plots reporting the relative abundance of each common off-target site in corresponding datasets obtained for different tested sgRNAs, as indicated. Boxed numbers indicate the Pearson’s correlation coefficient for the corresponding pair of experiments and the associated p-value (paired two-sided Wilcoxon signed-rank test). Sample sizes, corresponding to common off-target sites: VEGFA2=97, VEGFA3=12, EMX1=9, FANCF2=11. The Venn diagrams show the intersection among the datasets of the off-target sites detected for each tested sgRNA in association to wild-type SpCas9, as indicated. Previously published datasets included in these analyses are Tsai et al.9, Kleinstiver et al.1 and Chen et al.10. R1 and R2 indicate two replicate datasets generated in the present study for the VEGFA3 site in combination with wild-type SpCas9.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Notes (PDF 2720 kb)
Supplementary Table 1
This tables contains the list of the mutants recovered from the yeast screening (XLSX 35 kb)
Supplementary Table 2
This table contains the list of the oligonucleotides used for cloning and error-prone PCRs (XLSX 10 kb)
Supplementary Table 3
This table contains a list of the sgRNA spacers used in this study and their relative target sites (XLSX 12 kb)
Supplementary Table 4
This table contains a list of the oligonucleotides used to amplify target genomic loci and relative off-target sites (XLSX 14 kb)
Supplementary Table 5
This table, which has multiple tabs, contains the summary of data distributions and statistical details related to the manuscript figures (XLSX 41 kb)
Supplementary Data 1
This table, which has multiple tabs, contains the summary of GUIDE-seq data (XLSX 547 kb)
Supplementary Data 2
This table, which has multiple tabs, contains the summary of normalized GUIDE-seq reads (XLSX 275 kb)
Supplementary Data 3
This table, which has multiple tabs, contains the summary of targeted deep-sequencing data (XLSX 52 kb)
Rights and permissions
About this article
Cite this article
Casini, A., Olivieri, M., Petris, G. et al. A highly specific SpCas9 variant is identified by in vivo screening in yeast. Nat Biotechnol 36, 265–271 (2018). https://doi.org/10.1038/nbt.4066
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.4066
This article is cited by
-
Eukaryotic-driven directed evolution of Cas9 nucleases
Genome Biology (2024)
-
Engineering Cas9: next generation of genomic editors
Applied Microbiology and Biotechnology (2024)
-
Enrichment strategies to enhance genome editing
Journal of Biomedical Science (2023)
-
Recent advances in CRISPR-based genome editing technology and its applications in cardiovascular research
Military Medical Research (2023)
-
A cleavage rule for selection of increased-fidelity SpCas9 variants with high efficiency and no detectable off-targets
Nature Communications (2023)