Abstract
Controlling the rate of softening to extend shelf life was a key target for researchers engineering genetically modified (GM) tomatoes in the 1990s, but only modest improvements were achieved. Hybrids grown nowadays contain 'non-ripening mutations' that slow ripening and improve shelf life, but adversely affect flavor and color. We report substantial, targeted control of tomato softening, without affecting other aspects of ripening, by silencing a gene encoding a pectate lyase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
Change history
14 September 2016
In the version of this article initially published, the volume and page numbers for reference 46 were incorrect. The error has been corrected in the HTML and PDF versions of the article.
References
Vincent, H. et al. Biol. Conserv. 167, 265–275 (2013).
Robinson, R.W. & Tomes, M.L. Genet. Coop. 18, 36–37 (1968).
Vrebalov, J. et al. Science 296, 343–346 (2002).
Kitagawa, M. et al. Physiol. Plant. 123, 331–338 (2005).
Seymour, G.B., Østergaard, L., Chapman, N.H., Knapp, S. & Martin, C. Annu. Rev. Plant Biol. 64, 219–241 (2013).
Martin, L.B.B. & Rose, J.K.C. J. Exp. Bot. 65, 4639–4651 (2014).
The Tomato Genome Consortium. Nature 485, 635–641 (2012).
Smith, C.J.S. et al. Nature 334, 724–726 (1988).
Sheehy, R.E., Kramer, M. & Hiatt, W.R. Proc. Natl. Acad. Sci. USA 85, 8805–8809 (1988).
Smith, C.J.S. et al. Plant Mol. Biol. 14, 369–379 (1990).
Tieman, D.M., Harriman, R.W., Ramamohan, G. & Handa, A.K. Plant Cell 4, 667–679 (1992).
Tieman, D.M. & Handa, A.K. Plant Physiol. 106, 429–436 (1994).
Hall, L.N. et al. Plant J. 3, 121–129 (1993).
Smith, D.L., Abbott, J.A. & Gross, K.C. Plant Physiol. 129, 1755–1762 (2002).
Brummell, D.A. et al. Plant Cell 11, 2203–2216 (1999).
Jiménez-Bermúdez, S. et al. Plant Physiol. 128, 751–759 (2002).
Besford, R.T. & Hobson, G.E. Phytochemistry 11, 2201–2205 (1972).
Abe, M., Katsumata, H., Komeda, Y. & Takahashi, T. Development 130, 635–643 (2003).
Yeats, T.H. & Rose, J.K.C. Plant Physiol. 163, 5–20 (2013).
Mravec, J. et al. Development 141, 4841–4850 (2014).
Willats, W.G.T. et al. J. Biol. Chem. 276, 19404–19413 (2001).
Jarvis, M.C., Briggs, S.P.H. & Knox, J.P. Plant Cell Environ. 26, 977–989 (2003).
Eshed, Y. & Zamir, D. Euphytica 79, 175–179 (1994).
Causse, M. et al. J. Fd. Sci. 75, S531–S541 (2010).
Ward, T.M. et al. Isolation of Plant Growth Substances 4 (Cambridge University Press, 1978).
Chapman, N.H. et al. Plant Physiol. 159, 1644–1657 (2012).
Marín-Rodríguez, M.C., Smith, D.L., Manning, K., Orchard, J. & Seymour, G.B. Plant Mol. Biol. 51, 851–857 (2003).
Collmer, A., Ried, J.L. & Mount, M.S. Methods Enzymol. 161, 329–335 (1988).
Andrews, S. FastQC: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
Trapnell, C., Pachter, L. & Salzberg, S.L. Bioinformatics 25, 1105–1111 (2009).
Langmead, B. & Salzberg, S.L. Nat. Methods 9, 357–359 (2012).
Risso, D., Schwartz, K., Sherlock, G. & Dudoit, S. BMC Bioinformatics 12, 480 (2011).
Robinson, M.D., McCarthy, D.J. & Smyth, G.K. Bioinformatics 26, 139–140 (2010).
Young, M.D., Wakefield, M.J., Smyth, G.K. & Oshlack, A. Genome Biol. 11, R14 (2010).
Halket, J.M. et al. J. Exp. Bot. 56, 219–243 (2005).
Fraser, P.D., Pinto, M.E., Holloway, D.E. & Bramley, P.M. Plant J. 24, 551–558 (2000).
Perez-Fons, L. et al. Sci. Rep. 4, 3859 (2014).
Bolger, A. et al. Nat. Genet. 46, 1034–1038 (2014).
Buttery, R.G., Teranishi, R. & Ling, L.C. J. Agric. Food Chem. 35, 540–544 (1987).
Blumenkrantz, N. & Asboe-Hansen, G. Anal. Biochem. 54, 484–489 (1973).
Dam, J. & Schuck, P. Methods Enzymol. 384, 185–212 (2004).
Schuck, P. et al. Analyst (Lond.) 139, 79–92 (2014).
Domozych, D.S., Serfis, A., Kiemle, S.N. & Gretz, M.R. Protoplasma 230, 99–115 (2007).
Livak, K.J. & Schmittgen, T.D. Methods 25, 402–408 (2001).
Nekrasov, V., Staskawicz, B., Weigel, D., Jones, J.D. & Kamoun, S. Nat. Biotechnol. 31, 691–693 (2013).
Bird, C. et al. Plant Mol. Biol. 11, 651–662 (1988).
Orfila, C. et al. Plant Physiol. 126, 210–221 (2001).
Acknowledgements
S.U. was funded by Ministry of Education of the Turkish Republic. The work was partly funded by BBSRC and Syngenta Seeds Ltd. through BBSRC 'stand-alone LINK' grants to P.D.F. and G.B.S. (BB/J015598/1 and BB/J016071/1). As part of the BBSRC grant, Syngenta staff (J.S., S.S., C.B. and D.R.) provided support with generating the transgenic plants, the bioinformatics analysis, the microscopy and writing the paper. G.B.S. and P.D.F. acknowledge support from EU project FP6 EUSOL and the European Cooperation in Science and Technology (COST) Action FA1106. D.D. was funded by US National Science Foundation grants NSF-MRI 1337280 and NSF-MRI 0922805. B.B.-U. and A.L.T.P. were funded by US National Science Foundation grants IOS 0957264 and IOS 0544504. J.R. was funded by a grant (IOS-1339287) from the Plant Genome Research Program of the US National Science Foundation. We acknowledge Syngenta Crop Protection, Research Triangle Park, North Carolina, USA, M. Franco for cDNA library preparation and J. Ni for RNASeq quality checks, read alignment and gene counting. We acknowledge J. Jones, V. Nekrasov and S. Kamoun, T.S.L. and The Gatsby Charitable Foundation for provision of the CRISPR–Cas 9 vectors. We also thank M. Bennett and J. Labavitch for useful discussions. COS488 was kindly provided by J. Mravec and W.G.T. Willats of the Department of Plant and Environmental Sciences of the University of Copenhagen.
Author information
Authors and Affiliations
Contributions
S.U., N.H.C., R.S., M.P., G.A., R.B.G., T.M.D.B., J.S., S.S., N.S., D.W., L.P., I.D.F., N.Y., B.B.-U., E.A.F., L.S., and D.S.D. performed the experiments and data analysis, and J.C. did the statistical analysis. G.B.S. conceived and directed the project and G.B.S., J.C., D.G., C.B., D.R., R.F., A.L.T.P., S.E.H., P.D.F., G.A.T., and J.K.C.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.S., S.S., C.B. and D.R. were full-time employees of Syngenta during the study.
Integrated supplementary information
Supplementary Figure 1 PL gene expression in tomato fruit.
Relative expression of five PL-coding genes measured in at least three fruit of each genotype (cv. Alisa Craig) at four stages of development, immature-1 (IM-1, 10 days post anthesis, dpa), immature-2 (IM-2, 20 dpa), mature green (MG, 31 dpa) and red ripe (RR, breaker +7 days). Individual fruit means shown as dots. Letters correspond to significant differences between stages (P<0.05, two-tailed t-test). Primers for the PL-coding and reference genes are provided in Supplementary Table 8. The method of Livak & Schmittgen 44 was used to account for differences in primer efficiency.
Supplementary Figure 2 Mechanical measures of pericarp texture of two additional independent PL::RNAi transgenic fruit lines.
a, Outer and inner pericarp texture based on measuring maximum load in red ripe fruit at breaker + 7 of two additional (to PL5 described in main text) homozygous independent PL transgenic lines (PL1 and PL9) in comparison with an azygous wild type (WT) control. Error bars are s.e.m. based on at least four fruits measured from each genotype represented by dots. b, key taste and colour metabolites in breaker + 7 fruits of the two homozygous independent PL transgenic lines (PL1 and PL9) in comparison with an azygous wild type control (WT). There were no significant (F-test; P>0.05) differences between the transgenic and WT lines, except for lycopene levels in PL 1 alone where a small, but significant (F-test; P=0.02), increase was apparent in comparison with the control. s.e.m based on four individual fruits for PL1 and five individual fruits for PL9 and three from the WT line. All plants used for the texture and metabolite analyses above were grown summer 2013.
Supplementary Figure 3 Comparison of mechanical measurement of pericarp fruit firmness in PL and PG transgenic lines.
a, Outer and b, inner pericarp maximum load of red ripe fruits (breaker + 7 days) from azygous wild type control (WT), the PL::RNAi line PL5 and an antisense polygalacturonase (PG) line where PG expression was suppressed to below 1% of normal levels. Error bars are s.e.m. There were eight fruits of each genotype. Dots represent plant means. There was no significant difference in pericarp firmness between the control and antisense PG lines. The PL::RNAi line was significantly (F-test; P<0.05) firmer than both PG and control lines.
Supplementary Figure 4 Physiochemical characteristics of WT and PL::RNAi fruits are indistinguishable
Physiochemical characteristics, a, weight, b, pericarp skin colour, c, total soluble solids of azygous wild type control (WT) and PL::RNAi (PL) red ripe fruits at breaker +7 days and, d, fruit ethylene production from orange ripe fruits at breaker + 4 days. Error bars are s.e.m. There were no significant (F-test; P>0.05) differences be WT and PL fruits. There were four fruits measured from genotype PL5 represented by dots.
Supplementary Figure 5 Metabolite profiles of azygous and PL::RNAi fruits are indistinguishable
a, Carotenoids, sugars, acid levels and, b, volatiles in azygous wild type control (WT) and PL::RNAi (PL) in red ripe fruit at breaker + 7 days (PL). Error bars are s.e.m. There were no significant (Ftest; P>0.05) differences be azygous and PL::RNAi fruits. Three fruits were measured from control and PL5 fruits represented by dots.
Supplementary Figure 6 Summary chart showing transcriptome profiles for wild type and PL::RNAi lines
Distribution of expressed genes by GO terms from azygous wild type control (WT) and PL::RNAi line PL5 orange ripe fruits(breaker + 4 days) and red ripe fruits (breaker + 7 days) illustrating that the transcriptome profile of the fruit in the two treatments was essentially identical. Three individual fruits were sampled at each stage of ripening for each genotype and analysis of the RNASeq data is presented in detail in Supplementary Tables 2,3,4,5,6,7 and in the Methods section.
Supplementary Figure 7 The cuticle wax profiles of wild type and PL::RNAi red ripe fruits at breaker + 7 days
The total amount of wax, a, and the levels of alkanes and triterpenoids, b, were not significantly different between the azygous wild type control (shaded) and PL::RNAi (clear) bars, as was the case for almost all individual wax compounds. The only statistically significant (P=0.05; two-tailed t-test) differences were in the abundance of 32 and 33 carbon alkanes, but the magnitude of the differences were too small to account for the differences in fruit firmness based on transpirational water loss. We conclude that the elevated expression of CER1, which is involved in cuticular alkane biosynthesis in the RNAi line did not have a major effect on cuticle function or composition. Error bars are s.e.m. Four individual fruits, represented by dots, were sampled from the azygous and PL::RNAi PL5 genotypes.
Supplementary Figure 8 Immunogold labelling with JIM5 recognising demethylated homogalacturonan in cell walls of wild type and PL::RNAi pericarp parenchyma cells
The sections were cut from the pericarp parenchyma cells of a, azygous wild type control (WT) or b, PL::RNAi line PL5 fruits harvested at red ripe breaker + 7 days. The density of labelling is greater in the PL::RNAi line consistent with reduced degradation of demethylated pectins in these fruits. The micrographs are representative of images taken from pericarp sections of three individual fruit in each treatment.
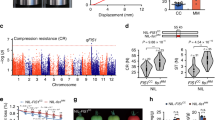
Supplementary Figure 9 Natural variation for fruit texture associated with PL
a, Fine mapping of the texture QTL on chromosome 3 and location of the PL locus (marker PLG690). Black bar shows QTL mapping interval of c.1 Mb between TG599 and G950, see Supplementary Figure 10. b, texture of the outer and,c, inner pericarp of fruit of the tomato cultivar M82, IL3-4 (in the M82 background and M82 x IL3-4 F1 fruits. d, PL gene expression at breaker + 7 in M82 and IL3-4. Error bars are s.e.m. For qRTPCR the means are based on three individual fruit (shown as dots) per genotype. Fruit texture measurements are based on at least 30 individual fruits for each genotype (n=32 for M82, 98 for F1, and 37 for IL3-4). Dots represent plant means. The texture data were obtained from tomato plants grown in Spring / Summer 2007.
Supplementary Figure 10 Physical structure of key recombinants showing markers used to delineate mapping interval for fruit firmness QTL on IL3-4
The fruit firmness QTL shown in Supplementary 9 is in a region of the tomato genome with suppressed recombination. The recombinants were identified and the mapping interval determined using markers derived from the tomato genome sequence (G- markers) or from the tomato genetic map (https://solgenomics.net/ and also see Supplementary Table 8). The numbers under each marker are the physical distance in bp along tomato chromosome 3 based on the tomato genome SLv2.50 build (https://solgenomics.net/). G690* is a marker in pectate lyase gene. TG599 defines the proximal end of the IL3-4 introgression (https://solgenomics.net/). The physical distance between TG599 and G950 = 1091323bp and between TG129 and G950 = 1785601bp. Lines possessing the M82 S.lycopersicum allele (pink) at G690 were significantly (P≰0.002) softer, in both the outer and inner pericarp tissues, than those with the IL3-4 S. pennellii allele (green) at the same position. The mean texture values for the outer and inner pericarp (two technical replicates for each measurement) for lines carrying the S. lycopersicum and S. pennellii alleles at G690 were compared by Analysis of Variance by fitting a linear mixed model with allelic variant as the fixed effect and plant, fruit within plant and technical replicate within fruit as random effects. This ensured that, within the analysis, the variation between means of plants having the different alleles at G690 was compared to the variation between plants of the same genotype at this position. Mean values for the outer pericarp were 0.528 and 1.204N for the lines with S. lycopersicum and S. pennellii alleles at G690, respectively (SED, 0.1267 with 6 df) and for the inner pericarp the values were 0.817 and 1.480N (SED 0.11862 with 6 df).
Supplementary Figure 11 CRISPR/Cas9-induced mutations in PL in transgenic tomato lines and analysis of pericarp texture and ripening properties
a, Target site for guide RNA (Cas9/sgRNA) used to edit the PL coding sequence by the protocol described in Nekrasov et al 45, thirteen transgenic plants harbouring the Cas9 gene were analysed by PCR and sequencing to identify mutations and the lines were then self –pollinated and homozygous mutants were identified by sequencing. Two CRISPR lines, PLC5 and PLC11, from independent transgenic events, yielded the same point mutation and the sequence is shown in comparison to wild type. The single bp insertion results in a stop codon in the PL coding sequence. b, Analysis of fruit from lines PLC5 and PLC11 harbouring the PL mutation and the azygous wild type control (WT) revealed fruits with significantly (Ftest; P≤0.04) firmer texture compared to the control as denoted by *. However there were no significant (Ftest; P> 0.05) differences in fruit colour or soluble solids content between PL and WT fruits. Error bars are s.e.m. Dots represent individual fruits. Fruit numbers were WT n=3, PLC11 n= 3, PLC 5 n=13.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 1117 kb)
Supplementary Table 1
Metabolite analysis PL=PLRNAi line PL5 and AZ = azygous control grown in Spring 2014. There were 3 individual fruits form each PL and AZ line with and 5 technical reps. (XLSX 23 kb)
Supplementary Table 2
Normalise RNASeq data for PL5 RNAi line and AZ BR4 and BR7 fruits 3 biological replicates. (XLSX 2057 kb)
Supplementary Table 3
Genes with > 2 fold up regulation in PL5 RNAi vs AZ at BR4 with 100 or more reads in PL RNAi lines. (XLSX 14 kb)
Supplementary Table 4
Genes with > 2 fold up regulation in PL5 RNAi vs AZ at BR7 with 100 or more reads in PL RNAi lines. (XLSX 19 kb)
Supplementary Table 5
Genes with > 2 fold down-regulation in PL5 RNAi vs AZ at BR4 with 100 or more reads in PL RNAi lines. (XLSX 15 kb)
Supplementary Table 6
Genes with > 2 fold down-regulation in PL5 RNAi vs AZ at BR7 with 100 or more reads in PL RNAi lines. (XLSX 18 kb)
Supplementary Table 7
Ripening-related cell wall structure genes showing significant differences in expression between PL5 RNAi and controls based on 2 fold changes. (XLSX 11 kb)
Supplementary Table 8
Primers for PCR amplification. (XLSX 10 kb)
Source data
Rights and permissions
About this article
Cite this article
Uluisik, S., Chapman, N., Smith, R. et al. Genetic improvement of tomato by targeted control of fruit softening. Nat Biotechnol 34, 950–952 (2016). https://doi.org/10.1038/nbt.3602
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3602
This article is cited by
-
Genome-edited foods
Nature Reviews Bioengineering (2023)
-
Tailoring crops with superior product quality through genome editing: an update
Planta (2023)
-
Salinity Stress Tolerance in Solanaceous Crops: Current Understanding and Its Prospects in Genome Editing
Journal of Plant Growth Regulation (2023)
-
The Tomato Juhwang Mutant Accumulates a High Level of β-Carotene and has a Long Shelf-Life
Journal of Plant Biology (2023)
-
An identical-by-descent segment harbors a 12-bp insertion determining fruit softening during domestication and speciation in Pyrus
BMC Biology (2022)