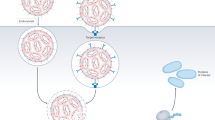
Abstract
The efficacy of cancer drugs is often limited because only a small fraction of the administered dose accumulates in tumors. Here we report an injectable nanoparticle generator (iNPG) that overcomes multiple biological barriers to cancer drug delivery. The iNPG is a discoidal micrometer-sized particle that can be loaded with chemotherapeutics. We conjugate doxorubicin to poly(L-glutamic acid) by means of a pH-sensitive cleavable linker, and load the polymeric drug (pDox) into iNPG to assemble iNPG-pDox. Once released from iNPG, pDox spontaneously forms nanometer-sized particles in aqueous solution. Intravenously injected iNPG-pDox accumulates at tumors due to natural tropism and enhanced vascular dynamics and releases pDox nanoparticles that are internalized by tumor cells. Intracellularly, pDox nanoparticles are transported to the perinuclear region and cleaved into Dox, thereby avoiding excretion by drug efflux pumps. Compared to its individual components or current therapeutic formulations, iNPG-pDox shows enhanced efficacy in MDA-MB-231 and 4T1 mouse models of metastatic breast cancer, including functional cures in 40–50% of treated mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Ferrari, M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 28, 181–188 (2010).
Michor, F., Liphardt, J., Ferrari, M. & Widom, J. What does physics have to do with cancer? Nat. Rev. Cancer 11, 657–670 (2011).
Lien, M.Y. et al. Safety and efficacy of pegylated liposomal doxorubicin-based adjuvant chemotherapy in patients with stage I-III triple-negative breast cancer. Anticancer Res. 34, 7319–7326 (2014).
Verma, S., Dent, S., Chow, B.J., Rayson, D. & Safra, T. Metastatic breast cancer: the role of pegylated liposomal doxorubicin after conventional anthracyclines. Cancer Treat. Rev. 34, 391–406 (2008).
Patel, H.M. & Moghimi, S.M. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system—the concept of tissue specificity. Adv. Drug Deliv. Rev. 32, 45–60 (1998).
Decuzzi, P. et al. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 141, 320–327 (2010).
Lesniak, A. et al. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 135, 1438–1444 (2013).
Gottesman, M.M., Fojo, T. & Bates, S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58 (2002).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Tasciotti, E. et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat. Nanotechnol. 3, 151–157 (2008).
Bae, Y., Fukushima, S., Harada, A. & Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Edn Engl. 42, 4640–4643 (2003).
Demaurex, N. pH Homeostasis of cellular organelles. News Physiol. Sci. 17, 1–5 (2002).
Padilla-Parra, S. et al. Quantitative imaging of endosome acidification and single retrovirus fusion with distinct pools of early endosomes. Proc. Natl. Acad. Sci. USA 109, 17627–17632 (2012).
Decuzzi, P., Pasqualini, R., Arap, W. & Ferrari, M. Intravascular delivery of particulate systems: does geometry really matter? Pharm. Res. 26, 235–243 (2009).
Godin, B. et al. Discoidal porous silicon particles: fabrication and biodistribution in breast cancer bearing mice. Adv. Funct. Mater. 22, 4225–4235 (2012).
van de Ven, A.L. et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J. Control. Release 158, 148–155 (2012).
Wike-Hooley, J.L., Haveman, J. & Reinhold, H.S. The relevance of tumour pH to the treatment of malignant disease. Radiother. Oncol. 2, 343–366 (1984).
Von Hoff, D.D. et al. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 91, 710–717 (1979).
Zhang, S. et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642 (2012).
Fojo, T. & Menefee, M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann. Oncol. 18 (suppl. 5), v3–v8 (2007).
Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 41, 189–207 (2001).
Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 91, 3–6 (2015).
Liedtke, C. et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 26, 1275–1281 (2008).
Shen, H. et al. Cooperative, nanoparticle-enabled thermal therapy of breast cancer. Adv. Healthc. Mater. 1, 84–89 (2012).
Alhareth, K., Vauthier, C., Gueutin, C., Ponchel, G. & Moussa, F. HPLC quantification of doxorubicin in plasma and tissues of rats treated with doxorubicin loaded poly(alkylcyanoacrylate) nanoparticles. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 887–888, 128–132 (2012).
Hurst, D.R. et al. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 69, 1279–1283 (2009).
Lu, X. & Kang, Y. Efficient acquisition of dual metastasis organotropism to bone and lung through stable spontaneous fusion between MDA-MB-231 variants. Proc. Natl. Acad. Sci. USA 106, 9385–9390 (2009).
Minn, A.J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
McConnell, K.I. et al. Enhanced gene delivery in porcine vasculature tissue following incorporation of adeno-associated virus nanoparticles into porous silicon microparticles. J. Control. Release 194, 113–121 (2014).
Acknowledgements
M.F. is grateful for the Ernest Cockrell Jr. Distinguished Endowed Chair in the Department of Nanomedicine at the Houston Methodist Research Institute. The authors acknowledge financial support from Department of Defense grants W81XWH-09-1-0212 and W81XWH-12-1-0414, National Institute of Health grants NIH U54CA143837, U54CA151668 (M.F.) and 1R01CA193880-01A1 (H.S.), Welch Foundation grant AU-1714 (J.L.), and National Natural Science Foundation of China 81301314 (R.X.). The authors thank the Nanofabrication Core of the Houston Methodist Research Institute for porous silicon microparticle fabrication and surface chemical modification. We acknowledge J. Gu for AFM analysis, D. Kirui for intravital microscopy, and H.H. Hoang for manuscript proofreading and editing.
Author information
Authors and Affiliations
Contributions
M.F. and H.S. developed the concept and supervised experiments. H.S., E.B. and M.F. prepared the manuscript with assistance from R.X., G.Z., X.D.; J.M., X.L. and Y.H. fabricated porous silicon particles. G.Z. and Z.H. synthesized pDox. G.Z., V.S.-I., S.W. and J.L. characterized iNPG-pDox. R.X., J.M., X.D., Z.H., J.S., Y.H., H.L., L.C. and E.K. performed in vivo biological analyses. J.E.E. performed biostatistical analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Synthesis and characterization of pDox.
(a) Synthesis of pDox. Chemical structure of the pDox prodrug is shown, and the pH-sensitive hydrazone linker is circled in red. (b) 1H-NMR demonstrating the chemical structure of pDox with characteristic peaks labeled. The reference spectrums from Dox and poly(L-glutamic acid) (PG) are on the right side.
Supplementary Figure 2 Scanning electron microscopic image of a porous silicon microparticle.
SEM image of an empty 2.5 μm × 700 nm discoidal porous silicon microparticle.
Supplementary Figure 3 Size measurement of pDox nanoparticles.
Dynamic light scattering histograms of (a) pDox nanoparticles released from iNPG and (b) self-assembled free pDox nanoparticles as control. Size distribution was measured by intensity. Note: A profile could not be obtained from supernatant of the iNPG control, as no particles were detectable. (c) AFM analysis on size distribution of released particles from iNPG at pH 6.6 and pH 5.2.
Supplementary Figure 4 Inhibition of triple negative breast cancer cell growth.
Cell viability of (a) MDA-MB-231 and (b) MDA-MB-468 after treatment for 96 hours with empty iNPG control, free Dox, pDox NP, or iNPG-pDox. (c) Structural comparison between pDox containing a pH-dependent hydrazone linker (highlighted in red) and amide-pDox without a hydrazine linker. (d) Cell viability of MDA-MB-231 and MDA-MB-468 cells after treatment for 96 hours with Dox, pDox NP, or amide-Dox.
Supplementary Figure 5 Uptake of pDox nanoparticles by MDA-MB-231 tumor cells.
(a) MDA-MB-231 tumor cells were treated with inhibitors of phagocytosis, macropinocytosis or endocytosis for 30 minutes before they were incubated with pDox for 2 hours. Cells internalized with the fluorescent pDox were identified with flow cytometry. **: p<0.01. (b) Intracellular trafficking of free Dox and pDox NP in MDA-MB-231 cells. Confocal microscopy was applied to visualize the red fluorescent drug in subcellular organelles. Nuclei were stained blue with DAPI and late endosomes/lysosomes were stained green with LysoTracker.
Supplementary Figure 6 H&E staining of heart tissue slides from nude mice treated once with 6 mg/kg Dox, Doxil, or iNPG-pDox.
Scale bar: 50 μm.
Supplementary Figure 7 Tissue biodistribution of iNPG-pDox in nude mice bearing lung metastatic MDA-MB-231 tumors.
(a) Tissue biodistribution of iNPG-pDox was evaluated based on silicon content analysis. Mice were intravenously dosed with 6 mg/kg iNPG-pDox and then euthanized at 1 hour, 1 day, or 1 week post treatment. Major organs were collected and processed for silicon content analysis. (b) Tissue biodistribution based on Dox content after an intraperitoneal injection of iNPG-pDox. Each mouse received 6 mg/kg iNPG-pDox. Drug content was measured in tissue samples collected 1 hour, 1 day, or 1 week after treatment.
Supplementary Figure 8 Tissue biodistribution based on Dox content in tumor-free nude mice after intravenous injection of Dox, pDox NP, or iNPG-pDox.
Each mouse received 6 mg/kg Dox, pDox NP, or iNPG-pDox. Differences and significance between iNPG-pDox and Dox or pDox NP were estimated by F-tests with Hommel’s adjustment of multiplicity.
Supplementary Figure 9 iNPG-pDox accumulation in lung metastatic tumor nodules.
(a) Intravital microscopic analysis on iNPG-pDox accumulation. Breast cancer lung metastasis was established with green fluorescent protein (GFP)-positive MDA-MB-231 cells. Mice were treated with intravenous injection of iNPG-pDox (red), and particle accumulation in the lung was visualized 1 hour and 24 hours after treatment. (b) H&E staining of lung tissue slides. Red arrows point to iNPG-pDox particles, and blue arrows point to red blood cells. Co-localization of iNPG-pDox and red blood cells indicates vascular localization of iNPG-pDox. (c) CD31 staining of lung tissue sections. Tumor vasculature was stained in brown, and red arrows point to iNPG-pDox.
Supplementary Figure 10 Apoptosis of tumor cells in vivo following iNPG-pDox treatment.
H&E stained tissue sections of tumor nodules in the lung from mice bearing metastatic MDAMB-231 tumors 24 h after administration with 6 mg/kg iNPG-pDox. Green arrows point to apoptotic bodies.
Supplementary Figure 11 Histological examination on inhibition of tumor growth in lung metastatic MDA-MB-231.
Mice with metastatic MDA-MB-231 tumors were treated every other week for 6 weeks. At the end of the treatments, mice were euthanized and the lung tissues were collected for (a) gross morphology analysis by H&E staining and (b) cell proliferation analysis by Ki-67 staining. Black arrows point to tumor nodules. Scale bars: 100 μm in (a) and 10 μm in (b).
Supplementary Figure 12 Accumulation of iNPG-pDox in lung metastatic 4T1 tumor nodules.
H&E staining of tissue sections of 4T1 lung metastatic tumors from a mouse treated once with 6 mg/kg iNPG-pDox. The green arrow points to iNPG-pDox. Scale bar: 10 μm.
Supplementary Figure 13 Inhibition of tumor growth and prolonged survival in mice bearing 4T1 tumor metastases to the liver and lung after treatment with iNPG-pDox.
(a) Bioluminescence monitoring of growth of 4T1 tumor metastasis following treatments. Mice (n = 10 mice/group) were treated weekly with 6 mg/kg Dox, Doxil, pDox NP, or iNPG-pDox for 4 weeks. Tumor growth was monitored by bioluminescence. Images of 5 mice per group are shown. (b) Kaplan-Meier plot of animal survival. Median survival time is listed in the table. Differences in survival were evaluated by the log-rank test. A global test demonstrated a difference exists among the groups. Pairwise comparisons were performed to evaluate the advantage of iNPG-pDox formulation over the clinical formulations. (c) Ki-67 staining of lung tumor nodules at the end of the 4-week treatments. Scale bar: 10 μm.
Supplementary Figure 14 Toxicity evaluation of iNPG-pDox.
(a) Toxicity evaluation of iNPG-pDox and Doxil in BALB/c and athymic nude mice (n = 3 mice/group). (b) Body weight changes in mice treated with 6 mg/kg, 12 mg/kg, and 24 mg/kg of iNPG-pDox and Doxil. (c) Complete blood count analysis in mice treated with iNPG-pDox and Doxil 6 days after treatment. The shadow area marks normal range. Nude mice treated with 24 mg/kg Doxil reached endpoint before analysis. Consequently, there was no data available for this treatment group. (d) Cardiac toxicity evaluation based on plasma LDL level in post-treatment mice. The shadow area marks normal range. No mice treated with 24 mg/kg Doxil were included, as they died before analysis.
Supplementary Figure 15 Mechanism of action of iNPG-pDox.
Sequential steps include vascular transport, tumor accumulation of iNPG-pDox in sites of interest due to innate tropism, association with diseased endothelia, in situ generation of pDox nanoparticles, and cellular internalization and transport of pDox that results in release of Dox in the perinuclear region inside the cell.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 and Supplementary Table 1 (PDF 1950 kb)
Rights and permissions
About this article
Cite this article
Xu, R., Zhang, G., Mai, J. et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol 34, 414–418 (2016). https://doi.org/10.1038/nbt.3506
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3506
This article is cited by
-
Preparation of FK-SA conjugate gel beads with double cross-linking for pH-controllable drug releasing
Polymer Bulletin (2023)
-
Recent advances in porous nanomaterials-based drug delivery systems for cancer immunotherapy
Journal of Nanobiotechnology (2022)
-
Synergetic therapy of glioma mediated by a dual delivery system loading α-mangostin and doxorubicin through cell cycle arrest and apoptotic pathways
Cell Death & Disease (2020)
-
Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy
Nature Communications (2019)
-
Polymers for extended-release administration
Biomedical Microdevices (2019)