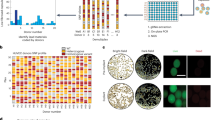
Abstract
The foreign body response is an immune-mediated reaction that can lead to the failure of implanted medical devices and discomfort for the recipient1,2,3,4,5,6. There is a critical need for biomaterials that overcome this key challenge in the development of medical devices. Here we use a combinatorial approach for covalent chemical modification to generate a large library of variants of one of the most widely used hydrogel biomaterials, alginate. We evaluated the materials in vivo and identified three triazole-containing analogs that substantially reduce foreign body reactions in both rodents and, for at least 6 months, in non-human primates. The distribution of the triazole modification creates a unique hydrogel surface that inhibits recognition by macrophages and fibrous deposition. In addition to the utility of the compounds reported here, our approach may enable the discovery of other materials that mitigate the foreign body response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
18 April 2016
In the version of this article initially published, one author, Adam C. Graham, his affiliation, and his contribution were omitted. In addition, two acknowledgments, to W. Salmon and J. Wyckoff, were omitted. The errors have been corrected in the HTML and PDF versions of the article.
References
Anderson, J.M., Rodriguez, A. & Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 (2008).
Langer, R. Perspectives and challenges in tissue engineering and regenerative medicine. Adv. Mater. 21, 3235–3236 (2009).
Ward, W.K. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2, 768–777 (2008).
Harding, J.L. & Reynolds, M.M. Combating medical device fouling. Trends Biotechnol. 32, 140–146 (2014).
Grainger, D.W. All charged up about implanted biomaterials. Nat. Biotechnol. 31, 507–509 (2013).
Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 29, 2941–2953 (2008).
Wick, G. et al. The immunology of fibrosis. Annu. Rev. Immunol. 31, 107–135 (2013).
Wynn, T.A. & Ramalingam, T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Zhang, L. et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556 (2013).
Sussman, E.M., Halpin, M.C., Muster, J., Moon, R.T. & Ratner, B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 42, 1508–1516 (2013).
Rodriguez, A., Meyerson, H. & Anderson, J.M. Quantitative in vivo cytokine analysis at synthetic biomaterial implant sites. J. Biomed. Mater. Res. A 89, 152–159 (2009).
Hetrick, E.M., Prichard, H.L., Klitzman, B. & Schoenfisch, M.H. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials 28, 4571–4580 (2007).
Ratner, B.D. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J. Control. Release 78, 211–218 (2002).
Lee, K.Y. & Mooney, D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 (2012).
Kearney, C.J. & Mooney, D.J. Macroscale delivery systems for molecular and cellular payloads. Nat. Mater. 12, 1004–1017 (2013).
Lim, F. & Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 210, 908–910 (1980).
Duvivier-Kali, V.F., Omer, A., Parent, R.J., O'Neil, J.J. & Weir, G.C. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes 50, 1698–1705 (2001).
de Vos, P., Faas, M.M., Strand, B. & Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 27, 5603–5617 (2006).
Tuch, B.E. et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32, 1887–1889 (2009).
Weir, G.C. Islet encapsulation: advances and obstacles. Diabetologia 56, 1458–1461 (2013).
Jacobs-Tulleneers-Thevissen, D. et al. Beta Cell Therapy Consortium EU-FP7. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia 56, 1605–1614 (2013).
Scharp, D.W. & Marchetti, P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv. Drug Deliv. Rev. 67-68, 35–73 (2014).
Robitaille, R. et al. Inflammatory response to peritoneal implantation of alginate-poly-L-lysine microcapsules. Biomaterials 26, 4119–4127 (2005).
Dang, T.T. et al. Enhanced function of immuno-isolated islets in diabetes therapy by co-encapsulation with an anti-inflammatory drug. Biomaterials 34, 5792–5801 (2013).
Rokstad, A.M. et al. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 7, 2566–2578 (2011).
King, A., Sandler, S. & Andersson, A. The effect of host factors and capsule composition on the cellular overgrowth on implanted alginate capsules. J. Biomed. Mater. Res. 57, 374–383 (2001).
Manoury, B., Caulet-Maugendre, S., Guénon, I., Lagente, V. & Boichot, E. TIMP-1 is a key factor of fibrogenic response to bleomycin in mouse lung. Int. J. Immunopathol. Pharmacol. 19, 471–487 (2006).
Brocchini, S., James, K., Tangpasuthadol, V. & Kohn, J. Structure-property correlations in a combinatorial library of degradable biomaterials. J. Biomed. Mater. Res. 42, 66–75 (1998).
Gu, M. et al. Combinatorial synthesis with high throughput discovery of protein-resistant membrane surfaces. Biomaterials 34, 6133–6138 (2013).
Bratlie, K.M. et al. Rapid biocompatibility analysis of materials via in vivo fluorescence imaging of mouse models. PLoS One 5, e10032 (2010).
Christen, T. et al. Molecular imaging of innate immune cell function in transplant rejection. Circulation 119, 1925–1932 (2009).
Haller, J. et al. Visualization of pulmonary inflammation using noninvasive fluorescence molecular imaging. J. Appl. Physiol. 104, 795–802 (2008).
Omer, A. et al. Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice. Diabetes 52, 69–75 (2003).
Omer, A. et al. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation 79, 52–58 (2005).
Veiseh, O. et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 14, 643–651 (2015).
Hofman, K., Hall, B., Cleaver, H. & Marshall, S. High-throughput quantification of hydroxyproline for determination of collagen. Anal. Biochem. 417, 289–291 (2011).
Paredes-Juarez, G.A., de Haan, B.J., Faas, M.M. & de Vos, P. A technology platform to test the efficacy of purification of alginate. Materials (Basel) 7, 2087–2103 (2014).
Paredes-Juarez, G.A., de Haan, B.J., Faas, M.M. & de Vos, P. The role of pathogen-associated molecular patterns in inflammatory responses against alginate based microcapsules. J. Control. Release 172, 983–992 (2013).
Sato, M. et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J. Immunol. 171, 417–425 (2003).
de Vos, P. et al. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials 30, 2559–2570 (2009).
Orive, G., Tam, S.K., Pedraz, J.L. & Hallé, J.P. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials 27, 3691–3700 (2006).
Dusseault, J. et al. Evaluation of alginate purification methods: effect on polyphenol, endotoxin, and protein contamination. J. Biomed. Mater. Res. A 76, 243–251 (2006).
Ménard, M. et al. Role of protein contaminants in the immunogenicity of alginates. J. Biomed. Mater. Res. B Appl. Biomater. 93, 333–340 (2010).
Madden, L.R. et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 107, 15211–15216 (2010).
Brauker, J.H. et al. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 29, 1517–1524 (1995).
Liu, M. et al. Stabilized hemocompatible coating of nitinol devices based on photo-cross-linked alginate/heparin multilayer. Langmuir 23, 9378–9385 (2007).
Kim, K., Cheng, J., Liu, Q., Wu, X.Y. & Sun, Y. Investigation of mechanical properties of soft hydrogel microcapsules in relation to protein delivery using a MEMS force sensor. J. Biomed. Mater. Res. A 92, 103–113 (2010).
de Haan, B.J. et al. Structural surface changes and inflammatory responses against alginate-based microcapsules after exposure to human peritoneal fluid. J. Biomed. Mater. Res. A 98, 394–403 (2011).
Qi, M. et al. A recommended laparoscopic procedure for implantation of microcapsules in the peritoneal cavity of non-human primates. J. Surg. Res. 168, e117–e123 (2011).
Kharb, R., Sharma, P.C. & Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzyme Inhib. Med. Chem. 26, 1–21 (2011).
Lindstedt, R. et al. The immunosuppressor st1959, a 3,5-diaryl-s-triazole derivative, inhibits T cell activation by reducing NFAT nuclear residency. Int. J. Immunopathol. Pharmacol. 22, 29–42 (2009).
Vegas, A.J. et al. Long term glycemic control using polymer-encapsulated human stem cell–derived beta cells in immune-competent mice. Nat. Med. 10.1038/nm.4030 (25 January 2016).
Stoppel, W.L. et al. Terminal sterilization of alginate hydrogels: efficacy and impact on mechanical properties. J. Biomed. Mater. Res. B Appl. Biomater. 102, 877–884 (2014).
Bernhardt, A. et al. Improved sterilization of sensitive biomaterials with supercritical carbon dioxide at low temperature. PLoS One 10, e0129205 (2015).
Kang, J.W. et al. Combined confocal Raman and quantitative phase microscopy system for biomedical diagnosis. Biomed. Opt. Express 2, 2484–2492 (2011).
Kang, J.W., Nguyen, F.T., Lue, N., Dasari, R.R. & Heller, D.A. Measuring uptake dynamics of multiple identifiable carbon nanotube species via high-speed confocal Raman imaging of live cells. Nano Lett. 12, 6170–6174 (2012).
Acknowledgements
This work was supported jointly by the JDRF and Leona M. and the Harry B. Helmsley Charitable Trust (grant 3-SRA-2014-285-M-R), National Institutes of Health (NIH grants EB000244, EB000351, DE013023 and CA151884), NIH NIBIB (P41EB015871-27), MIT SkolTech initiative (J.W.K.), JDRF and the Department of Defense/Congressionally Directed Medical Research Programs (DOD/CDMRP postdoctoral fellowships 3-2013-178 and W81XWH-13-1-0215 for O.V.) and through a generous gift from the Tayebati Family Foundation. G.C.W. is supported by National Institutes of Health (NIH grants R01DK093909 and P30DK036836, the Joslin Diabetes Research Center and its Advanced Microscopy Core), as well as the Diabetes Research and Wellness Foundation. J.O. is supported by the National Institutes of Health (NIH/NIDDK) R01DK091526 and the Chicago Diabetes Project. This work was also supported in part by the Koch Institute Support (core) grant P30-CA14051 from the National Cancer Institute. We also thank the Koch Institute Swanson Biotechnology Center for technical support, specifically Tang Histology Facility, Microscopy, Flow Cytometry, Nanotechnology Materials, and Applied Therapeutics and Whole Animal Imaging. The authors would like to acknowledge the use of resources at the Harvard University Center for Nanoscale Systems and W.M. Keck Biological Imaging Facility (Whitehead Institute). The authors would also like to thank W. Salmon and J. Wyckoff for their assistance.
Author information
Authors and Affiliations
Contributions
A.J.V., O.V., J.C.D., M.M., K.B., J.L., A.R.B., E.L., K.O., P.F., J.W.K., J.H.-L., M.A.B., A.C., S.S., K.T., S.J., S.A.-D., N.D., R.T., T.V., M.C., J.C., K.S., M.Q. and J.M. designed and performed experiments, and analyzed data. H.H.T. assisted with data processing and data presentation. A.C.G. assisted with SEM imaging. S.L. assisted with histology. D.M.H., D.L.G., J.O. and G.C.W. provided conceptual advice and technical support. A.J.V. and D.G.A. wrote the paper. R.L. and D.G.A. supervised the study. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.J.V., O.V., J.C.D., M.M., K.B., R.L. and D.G.A. declare financial interest in patents filed by MIT on the material and hydrogel capsule technology.
Integrated supplementary information
Supplementary Figure 1 Reagents used for polymer modification.
Amines, alcohols, azides, and alkynes used for the chemical modification of alginate.
Supplementary Figure 2 Additional data for lead materials tested in C57BL/6J mice.
(a) Evaluation of enzyme activity of cathespins B and L in the absence and presence of 20 mM barium chloride. No reduction in enzymatic activity is observed (n = 6, mean values ± SD). (b) Representative hematoxylin and eosin (HE) stained subcutaneous 28 day histology of the top ten alginate analogue microcapsules and control alginate microcapsules (SLG20, V/S) that were implanted in Figure 2, n = 3 mice per group. Abnormal microcapsule morphology is caused by histological processing (dehydration) of the tissue. Scale bar = 400 µm. (c) PCA functional group analysis of the entire modified alginate library, with top performing materials indicated. Triazole-containing modifications are enriched for favorable in vivo performance. (d) Western-blot images used for SMA quantification in Figure 2c. Protein was extracted from retrieved microcapsules (n = 5 mice per group) of the top three alginate analogues and controls after 14 days IP in C57BL/6J mice. (e) Cellular viability of RAW 264.7 cells exposed to formulated lead microcapsules (n = 12, mean values ± SD). (f) Cytokine panel analysis of protein extracted from retrieved microcapsules (n = 5 mice per group) in Figure 2a. Scale shows average fold signal above background.
Supplementary Figure 3 Nanostring gene expression analysis.
Gene expression analysis of seventy-nine known inflammatory factors and immune cell markers from retrieved IP fluid, fat pad, and microcapsules 14 days post-implantation in C57BL/6J mice. Gene expression from IP and fat pad was normalized to mock implant controls, while gene expression from microcapsule-associated tissue was normalized to the SLG20 sample.
Supplementary Figure 4 Determination of potential contaminants in controls and lead materials.
(a) Determination of potential contaminant levels of alginate microcapsules prior to implantation. Endotoxin and glucan levels were measured and reported by Charles River Laboratories. The standard curves used for quantification of (b) flagellin and (c) LTA levels are also shown.
Supplementary Figure 5 Additional FACS data.
FACS analysis of (a) macrophages and (b) neutrophils isolated from retrieved 300 µm microcapsules after 14 days IP in C57BL/6J mice, n = 5 mice per group. One-way ANOVA with Bonferroni correction was utilized to allow for statistical comparison of multiple means, # = p < 0.05, ** = p < 0.001, *** = p < 0.0001, ns = not signficant. The data express the number of cells as a percentage of total cells measured. (c) Representative dot plots of FACS analysis for macrophages and neutrophils presented in (a) and (b).
Supplementary Figure 6 Additional confocal raman and cryo-SEM imaging of lead materials.
(a) Confocal raman cross-section mapping of 300 µm Z1-Y15 microcapsules. The raman peak at 857 cm-1 (shown in red) is indicative of the thiomorpholine dioxide end group of Z1-Y15, and peak intensity is enriched at the surface of the microcapsules than at the core. The peak at 884 cm-1 is mapped in green as a reference to the alginate backbone structure. (b) Confocal raman cross-section mapping of 300 µm Z1-Y19 microcapsules. The raman peak at 1563 cm-1 (shown in red) is indicative of the aniline end group of Z1-Y19, and peak intensity appears more uniform at both the surface of the microcapsules and at the core. The peak at 884 cm-1 is mapped in green as a reference to the alginate backbone structure. (c) Representative freeze-fracture cryo-SEM images of the core (scale bar = 10 µm) and fractured surface (scale bar = 10 µm) of 300 µm SLG20, V/S, Z2-Y12, Z1-Y15, and Z1-Y19 microcapsules.
Supplementary Figure 7 NHP omental histology and western blot images of lead material implants.
(a) Representative MT and HE stained histology of biopsied omental tissue 4 weeks post-implantation in cynomolgus macaque, n = 3. (b) Western-blot images used for SMA quantification of protein extracted from the top three alginate analogue spheres and control spheres (n = 3).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Table 1 and Supplementary Note (PDF 2082 kb)
Rights and permissions
About this article
Cite this article
Vegas, A., Veiseh, O., Doloff, J. et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol 34, 345–352 (2016). https://doi.org/10.1038/nbt.3462
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3462
This article is cited by
-
Inflammation-induced subcutaneous neovascularization for the long-term survival of encapsulated islets without immunosuppression
Nature Biomedical Engineering (2023)
-
Immunization against Zika by entrapping live virus in a subcutaneous self-adjuvanting hydrogel
Nature Biomedical Engineering (2023)
-
Screening hydrogels for antifibrotic properties by implanting cellularly barcoded alginates in mice and a non-human primate
Nature Biomedical Engineering (2023)
-
Advancing cardiac regeneration through 3D bioprinting: methods, applications, and future directions
Heart Failure Reviews (2023)
-
A Simple Mathematical Model Demonstrates the Potential for Cell-Based Hormone Therapy to Address Dysregulation of the Hypothalamus-Pituitary-Ovary Axis in Females with Loss of Ovarian Function
Annals of Biomedical Engineering (2023)