Abstract
There are numerous cell types with scarcely understood functions, whose interactions with the immune system are not well characterized. To facilitate their study, we generated a mouse bearing enhanced green fluorescent protein (EGFP)-specific CD8+ T cells. Transfer of the T cells into EGFP reporter animals can be used to kill EGFP-expressing cells, allowing selective depletion of desired cell types, or to interrogate T-cell interactions with specific populations. Using this system, we eliminate a rare EGFP-expressing cell type in the heart and demonstrate its role in cardiac function. We also show that naive T cells are recruited into the mouse brain by antigen-expressing microglia, providing evidence of an immune surveillance pathway in the central nervous system. The just EGFP death-inducing (Jedi) T cells enable visualization of a T-cell antigen. They also make it possible to utilize hundreds of existing EGFP-expressing mice, tumors, pathogens and other tools, to study T-cell interactions with many different cell types, to model disease states and to determine the functions of poorly characterized cell populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pamer, E. & Cresswell, P. Mechanisms of MHC class I–restricted antigen processing. Annu. Rev. Immunol. 16, 323–358 (1998).
Fletcher, A.L., Malhotra, D. & Turley, S.J. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol. 32, 12–18 (2011).
Mueller, S.N. & Germain, R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 9, 618–629 (2009).
Kisielow, P., Blüthmann, H., Staerz, U.D., Steinmetz, M. & von Boehmer, H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333, 742–746 (1988).
Clarke, S.R. et al. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78, 110–117 (2000).
Lafaille, J.J. T-cell receptor transgenic mice in the study of autoimmune diseases. J. Autoimmun. 22, 95–106 (2004).
Berg, L.J. et al. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell 58, 1035–1046 (1989).
Saito, M. et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat. Biotechnol. 19, 746–750 (2001).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Schmidt, E.F., Kus, L., Gong, S. & Heintz, N. BAC transgenic mice and the GENSAT database of engineered mouse strains. Cold Spring Harb. Protoc. doi:10.1101/pdb.top073692 (1 March 2013).
Kirak, O. et al. Transnuclear mice with predefined T cell receptor specificities against Toxoplasma gondii obtained via SCNT. Science 328, 243–248 (2010).
Gambotto, A. et al. Immunogenicity of enhanced green fluorescent protein (EGFP) in BALB/c mice: identification of an H2-Kd-restricted CTL epitope. Gene Ther. 7, 2036–2040 (2000).
Norbury, C.C., Malide, D., Gibbs, J.S., Bennink, J.R. & Yewdell, J.W. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3, 265–271 (2002).
Agudo, J. et al. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 15, 54–62 (2014).
Hara, M. et al. Transgenic mice with green fluorescent protein-labeled pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 284, E177–E183 (2003).
Engelhardt, B. & Ransohoff, R.M. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 33, 579–589 (2012).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Ransohoff, R.M. & Cardona, A.E. The myeloid cells of the central nervous system parenchyma. Nature 468, 253–262 (2010).
Sándor, N. et al. Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice. PLoS One 9, e112397 (2014).
Ajami, B., Bennett, J.L., Krieger, C., McNagny, K.M. & Rossi, F.M.V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014).
Kim, J.M., Rasmussen, J.P. & Rudensky, A.Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Fontenot, J.D. et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22, 329–341 (2005).
Wu, M., Peng, S. & Zhao, Y. Inducible gene deletion in the entire cardiac conduction system using Hcn4-CreERT2 BAC transgenic mice. Genesis 52, 134–140 (2014).
Herrmann, S., Hofmann, F., Stieber, J. & Ludwig, A. HCN channels in the heart: lessons from mouse mutants. Br. J. Pharmacol. 166, 501–509 (2012).
Bucchi, A., Barbuti, A., Difrancesco, D. & Baruscotti, M. Funny current and cardiac rhythm: insights from HCN knockout and transgenic mouse models. Front. Physiol. 3, 240 (2012).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003).
Parish, I.A. et al. Tissue destruction caused by cytotoxic T lymphocytes induces deletional tolerance. Proc. Natl. Acad. Sci. USA 106, 3901–3906 (2009).
Egli, D. & Eggan, K. Nuclear transfer into mouse oocytes. J. Vis. Exp. 1, 116 (2006).
Van Thuan, N. et al. The histone deacetylase inhibitor scriptaid enhances nascent mRNA production and rescues full-term development in cloned inbred mice. Reproduction 138, 309–317 (2009).
Brown, B.D. et al. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat. Biotechnol. 25, 1457–1467 (2007).
Acknowledgements
We thank A. Annoni, L. Sherman, J. Brody, J. Lafaille, S. Lira, J. Blander and M. Feldmann for helpful discussions. We also thank K. Kelly and the Mouse Genetics and Mouse Targeting facility, and A. Rahman and the Flow Cytometry Core for technical assistance. B.D.B. and M.M. were supported by US National Institutes of Health (NIH) R01AI104848 and R01AI113221. B.D.B. was also supported by a Pilot Award from the Diabetes Research, Obesity and Metabolism Institute and an Innovation Award from the Juvenile Diabetes Research Foundation (JDRF). Y.Z. was supported by NIH1R01HL107376. J.A. was supported by the Robin Chemers Neustein Award, and a JDRF postdoctoral fellowship. V.K. was supported by a Swiss National Foundation Early Postdoc Mobility fellowship.
Author information
Authors and Affiliations
Contributions
J.A. designed and performed experiments, analyzed data and wrote the manuscript, A.R., E.S.P., R.S. M.W. and V.K. performed experiments or acquired data, Y.Z. analyzed data, D.E. performed experiments, M.M. designed experiments, analyzed data, and edited the manuscript, B.D.B. designed and supervised the research, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Immune cell characterization of the Jedi mice and sequence of the Jedi T cell receptor.
(a) Brachial lymph nodes (bLN), spleen, bone marrow (BM), liver, lung and thymus from Jedi mice and control littermates were stained with CD3e, CD4, CD8a antibodies, and an H-2Kd-GFP200-208 pentamer to measure the frequency of EGFP-specific T cells. bLN, spleen, BM, liver and lung were also stained with CD62L and CD44 antibodies to determine the percentage of naïve and memory CD8 T cells. Graphs present the mean±s.d. of the frequency of CD8+ T cells relative to the total live cells in bLN and spleen from the Jedi mice. Data are representative of 2 and 3 experiments (n=4-8 mice/group). (b) Graphs show the percentage of CD8+ T cells compared to total live or total CD3e+ cells in the bLN, spleen, BM, liver and lung (n=4-8 mice/group). *P<0.05 vs WT, **P<0.01 vs WT.
Supplementary Figure 2 Jedi T cells specifically recognize EGFP
(a) Sequence of the rearranged alpha and beta chains in the Jedi mice. (b) Comparison of the amino acid sequence between similar fluorescent proteins, namely Yellow Fluorescent Protein (eYFP), mCitrine, Green Fluorescent Protein (eGFP) and mCerulean. Highlighted in bold is the immune epitope of eGFP. (c) Bone marrow cells were transduced with a lentivirus expressing EYFP or EGFP and cultured in the presence GM-CSF to generate dendritic cells (BMDCs). Isolated Jedi or B10D2 CD8+ T cells were stained with Violet Cell Proliferation dye and added to BMDCs. EGFP-specific cells were identified by H-2Kd-GFP200-208 pentamer staining. Graph present the mean±s.d. of the frequency of cells that have proliferated (n=3 wells/group). ***P<0.001 vs each group.
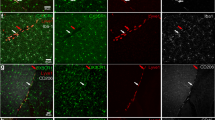
Supplementary Figure 3 Jedi T cells cross the blood brain barrier and kill EGFP-expressing microglia.
(a) Blood from bone marrow transplanted CX3CR1-EGFP mice was obtained 10 weeks after the transplant to confirm bone marrow engraftment prior to the transfer of the Jedi T cells. (b) Representative flow cytometry plots showing EGFP expression in the bone marrow (BM) of BM transplanted CX3CR1-EGFP mice 1 week after transferring Jedi T cells. (c) Representative flow cytometry plots showing EGFP expression in the lymph nodes (LN) of BM transplanted CX3CR1-EGFP mice 1 week after transferring Jedi T cells. (d) Representative images of the brain showing EGFP expression (signifying microglia) on tissue sections in different regions of the brain (20x magnification, bar represents 50 µm). (e) Representative flow cytometry plots showing CD45.1 Ctrl or Jedi T cells in the brain of BM transplanted CX3CR1-EGFP mice 1 week after transferring the indicated type of T cells. Graphs show the percentage of CD45.1+ cells compared to total CD45+ cells and the total number of CD45.1+ cells in the brain (n=3 mice/group). ***P<0.001 vs Control-treated. (f) Representative images of the brain showing EGFP expression (signifying microglia) and T cells (by CD3 staining) on tissue sections (20x magnification, bar represents 50 µm).
Supplementary Figure 4 Jedi T cell can stably deplete a renewing cell population and can be used to phenocopy Foxp3-DTR mice.
(a) Foxp3-EGFP male mice were injected with either control (Ctrl) or Jedi T cells. Percentage of EGFP+ cells among CD4 T cells was determined in the blood at the indicated time points (n=3-4 mice/group). **P<0.01 vs Control-treated, ***P<0.01 vs Control-treated. (b) Percentage of Jedi T cells among CD8+ T cells was determined in the blood at the indicated time points from the mice described in (a) (n=3-4 mice/group). The Jedi mice and control littermates were CD45.1 while the recipient Foxp3-EGFP mice were CD45.2, which permitted the cells to be detected by flow cytometry analysis. (c) Representative flow cytometry plots showing neutrophils in the blood of Ctrl or Jedi-treated male Foxp3-EGFP mice 9 days after the treatment. Graphs show the percentage of neutrophils (CD11b+ Gr1hi SSC-Ahi) compared to total live cells in the blood (n=3-4 mice/group). ***P<0.001 vs Control-treated. (d) Male Foxp3-EGFP mice were injected with either 5x104, (blue), 1.5x105 (red), or 5x105 (green) RFP+ CD8+ Jedi T cells and vaccinated against EGFP. The percentage of EGFP+ cells among CD4 T cells was determined in the blood at the indicated time points. The graph represents the percentage of GFP+/CD4+ cells compared to the initial time point prior to the injection of the T cells at day 0. Each line represents one mouse. (e) Percentage of CD8+ Jedi T cells among total CD8+ T cells was determined in the blood at the indicated time points from the mice described in (d). The Jedi mice were RFP+, which permitted the cells to be detected by flow cytometry analysis. Each line represents one mouse.
Supplementary Figure 5 Jedi T cells can deplete rare cell populations to study their function.
Electrocardiograms (ECG) from additional mice treated as described in Figure 4. Images are representative of 2 experiments (n=4 mice/group).
Supplementary Figure 6 Gating strategies for flow cytometry analysis.
(a) Representative gating strategy of the spleen of B10D2 mice after injection of either LV.GFP or LV.YFP and adoptive transfer of either control or Jedi T cells. This gating strategy was used in Figure 1c and 1e. (b) Representative gating strategy of the in vitro cultures of BM-DCs and T cells to determine T cell proliferation. This gating strategy was followed in Supplementary Figure 2c. (c) Representative gating strategy of the brain from CX3CR1-GFP mice after adoptive transfer of either control or Jedi T cells. This gating strategy was used in Figure 2. (d) Representative gating strategy of the blood from FoxP3-GFP mice after adoptive transfer of either RFP+ control or RFP+ Jedi T cells. This gating strategy was used in Figure 3b and c. (e) Representative gating strategy of the heart from Hcn4-GFP mice after adoptive transfer of either control or Jedi T cells. This gating strategy was used in Figure 4d.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1-6 (PDF 8732 kb)
Supplementary Table
Supplementary Table 1 (XLSX 81 kb)
Rights and permissions
About this article
Cite this article
Agudo, J., Ruzo, A., Park, E. et al. GFP-specific CD8 T cells enable targeted cell depletion and visualization of T-cell interactions. Nat Biotechnol 33, 1287–1292 (2015). https://doi.org/10.1038/nbt.3386
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3386
This article is cited by
-
Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity
Nature Communications (2022)
-
Proteogenomic discovery of neoantigens facilitates personalized multi-antigen targeted T cell immunotherapy for brain tumors
Nature Communications (2021)
-
Inducible de novo expression of neoantigens in tumor cells and mice
Nature Biotechnology (2021)
-
Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells
Nature Protocols (2017)
-
Jedi cells patrol the mouse
Nature Methods (2016)