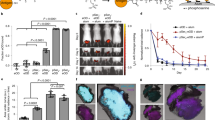
Abstract
The efficacy of vaccine adjuvants such as Toll-like receptor agonists (TLRa) can be improved through formulation and delivery approaches. Here, we attached small molecule TLR-7/8a to polymer scaffolds (polymer–TLR-7/8a) and evaluated how different physicochemical properties of the TLR-7/8a and polymer carrier influenced the location, magnitude and duration of innate immune activation in vivo. Particle formation by polymer–TLR-7/8a was the most important factor for restricting adjuvant distribution and prolonging activity in draining lymph nodes. The improved pharmacokinetic profile by particulate polymer–TLR-7/8a was also associated with reduced morbidity and enhanced vaccine immunogenicity for inducing antibodies and T cell immunity. We extended these findings to the development of a modular approach in which protein antigens are site-specifically linked to temperature-responsive polymer–TLR-7/8a adjuvants that self-assemble into immunogenic particles at physiologic temperatures in vivo. Our findings provide a chemical and structural basis for optimizing adjuvant design to elicit broad-based antibody and T cell responses with protein antigens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17, 1055–1065 (2010).
Epstein, J.E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334, 475–480 (2011).
Andersen, P. & Woodworth, J.S. Tuberculosis vaccines—rethinking the current paradigm. Trends Immunol. 35, 387–395 (2014).
Arens, R., van Hall, T., van der Burg, S.H., Ossendorp, F. & Melief, C.J. Prospects of combinatorial synthetic peptide vaccine-based immunotherapy against cancer. Semin. Immunol. 25, 182–190 (2013).
Guy, B. The perfect mix: recent progress in adjuvant research. Nat. Rev. Microbiol. 5, 505–517 (2007).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Nordly, P., Madsen, H.B., Nielsen, H.M. & Foged, C. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin. Drug Deliv. 6, 657–672 (2009).
Brito, L.A. & O'Hagan, D.T. Designing and building the next generation of improved vaccine adjuvants. J. Control. Release 190C, 563–579 (2014).
Fox, C.B. et al. TLR4 ligand formulation causes distinct effects on antigen-specific cell-mediated and humoral immune responses. Vaccine 31, 5848–5855 (2013).
Wille-Reece, U. et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. USA 102, 15190–15194 (2005).
Shukla, N.M. et al. Toward self-adjuvanting subunit vaccines: model peptide and protein antigens incorporating covalently bound toll-like receptor-7 agonistic imidazoquinolines. Bioorg. Med. Chem. Lett. 21, 3232–3236 (2011).
Wu, C.C. et al. Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc. Natl. Acad. Sci. USA 104, 3990–3995 (2007).
Kasturi, S.P. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011).
Jewell, C.M., Lopez, S.C. & Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 108, 15745–15750 (2011).
Ilyinskii, P.O. et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine 32, 2882–2895 (2014).
Moon, J.J. et al. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. USA 109, 1080–1085 (2012).
Smirnov, D., Schmidt, J.J., Capecchi, J.T. & Wightman, P.D. Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine 29, 5434–5442 (2011).
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014).
Wu, T.Y. et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 6, 263ra160 (2014).
Bachmann, M.F. & Jennings, G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 (2010).
Manolova, V. et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 38, 1404–1413 (2008).
Reddy, S.T., Rehor, A., Schmoekel, H.G., Hubbell, J.A. & Swartz, M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 112, 26–34 (2006).
Kourtis, I.C. et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS ONE 8, e61646 (2013).
Gorden, K.B. et al. Synthetic TLR Agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 174, 1259–1268 (2005).
Oh, J.Z., Kurche, J.S., Burchill, M.A. & Kedl, R.M. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood 118, 3028–3038 (2011).
Coffman, R.L., Sher, A. & Seder, R.A. Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503 (2010).
Gerster, J.F. et al. Synthesis and structure-activity-relationships of 1H-imidazo[4,5-c]quinolines that induce interferon production. J. Med. Chem. 48, 3481–3491 (2005).
A, A.G., Tyring, S.K. & Rosen, T. Beyond a decade of 5% imiquimod topical therapy. J. Drugs Dermatol. 8, 467–474 (2009).
Savage, P. et al. A phase I clinical trial of imiquimod, an oral interferon inducer, administered daily. Br. J. Cancer 74, 1482–1486 (1996).
Pockros, P.J. et al. Oral resiquimod in chronic HCV infection: safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J. Hepatol. 47, 174–182 (2007).
Seymour, L.W. et al. Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 20, 1668–1676 (2002).
Liu, X.M., Miller, S.C. & Wang, D. Beyond oncology—application of HPMA copolymers in non-cancerous diseases. Adv. Drug Deliv. Rev. 62, 258–271 (2010).
Seymour, L.W., Duncan, R., Strohalm, J. & Kopecek, J. Effect of molecular weight (Mbarw) of N-(2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal, and intravenous administration to rats. J. Biomed. Mater. Res. 21, 1341–1358 (1987).
Sharp, F.A. et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. USA 106, 870–875 (2009).
Russo, C. et al. Small molecule Toll-like receptor 7 agonists localize to the MHC class II loading compartment of human plasmacytoid dendritic cells. Blood 117, 5683–5691 (2011).
Ryu, K.A., Stutts, L., Tom, J.K., Mancini, R.J. & Esser-Kahn, A.P. Stimulation of innate immune cells by light-activated TLR7/8 agonists. J. Am. Chem. Soc. 136, 10823–10825 (2014).
Shakya, A.K., Holmdahl, R., Nandakumar, K.S. & Kumar, A. Characterization of chemically defined poly-N-isopropylacrylamide based copolymeric adjuvants. Vaccine 31, 3519–3527 (2013).
Shi, H.S. et al. Novel vaccine adjuvant LPS-Hydrogel for truncated basic fibroblast growth factor to induce antitumor immunity. Carbohydr. Polym. 89, 1101–1109 (2012).
Chou, H.Y. et al. Hydrogel-delivered GM-CSF overcomes nonresponsiveness to hepatitis B vaccine through the recruitment and activation of dendritic cells. J. Immunol. 185, 5468–5475 (2010).
Blander, J.M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006).
Nair-Gupta, P. et al. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell 158, 506–521 (2014).
Jing, P., Rudra, J.S., Herr, A.B. & Collier, J.H. Self-assembling peptide-polymer hydrogels designed from the coiled coil region of fibrin. Biomacromolecules 9, 2438–2446 (2008).
Pechar, M. & Pola, R. The coiled coil motif in polymer drug delivery systems. Biotechnol. Adv. 31, 90–96 (2013).
Kastenmüller, K. et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J. Clin. Invest. 121, 1782–1796 (2011).
Vecchi, S. et al. Conjugation of a TLR7 agonist and antigen enhances protection in the S. pneumoniae murine infection model. Eur. J. Pharm. Biopharm. 87, 310–317 (2014).
Scott, E.A. et al. Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes. Biomaterials 33, 6211–6219 (2012).
Liu, H., Kwong, B. & Irvine, D.J. Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angew. Chem. Intl. Edn. Engl. 50, 7052–7055 (2011).
Krieg, A.M. & Stein, C.A. Phosphorothioate oligodeoxynucleotides: antisense or anti-protein? Antisense Res. Dev. 5, 241 (1995).
Lahoud, M.H. et al. DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc. Natl. Acad. Sci. USA 109, 16270–16275 (2012).
de Titta, A. et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA 110, 19902–19907 (2013).
Shukla, N.M., Malladi, S.S., Mutz, C.A., Balakrishna, R. & David, S.A. Structure-activity relationships in human toll-like receptor 7-active imidazoquinoline analogs. J. Med. Chem. 53, 4450–4465 (2010).
Hruby, M. et al. New bioerodable thermoresponsive polymers for possible radiotherapeutic applications. J. Control. Release 119, 25–33 (2007).
Subr, V. & Ulbrich, K. Synthesis and properties of new N-(2-hydroxypropyl)-methacrylamide copolymers containing thiazolidine-2-thione reactive groups. React. Funct. Polym. 66, 1525–1538 (2006).
Skeiky, Y.A. et al. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine 20, 3292–3303 (2002).
Gerner, M.Y., Kastenmuller, W., Ifrim, I., Kabat, J. & Germain, R.N. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37, 364–376 (2012).
Quinn, K.M. et al. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 190, 2720–2735 (2013).
Darrah, P.A. et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13, 843–850 (2007).
Seder, R.A., Darrah, P.A. & Roederer, M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8, 247–258 (2008).
Acknowledgements
The authors wish to acknowledge M. Dillon, K. Wuddie and C. Chiedi at the Vaccine Research Center and B. Klaunberg and V. Diaz at the Mouse Imaging Facility (MIF) for their valuable support and assistance with the animal studies. We would also like to thank K. Ulbrich, R. Swenson and G. Griffiths for their support and helpful insights. This work was supported in part by the BIOPOL project (Grant of the Ministry of Education, Youth and Sports of the Czech Republic, no. EE2.3.30.0029); by the Czech Science Foundation (15-15181S); by Charles University (UNCE 204025/2012); by a Cancer Research UK grant (C552/A17720); and by the Office of AIDS Research and the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
G.M.L., R.L., K.D.F., L.W.S. and R.A.S were involved in experimental planning, interpreting data and writing the manuscript. G.M.L., R.L., A.E.D., O. Vasalatiy, J.K.T., L.S., K.A.R. and A.P.E.-K. planned and carried out the synthesis, purification and characterization of small molecules. R.L., R.P., M.P., T.E., O. Vanek and G.M.L. planned and completed the synthesis, purification and characterization of the polymer precursors and polymer conjugates. P.A.D, A.S.I., A.J.B., A.Y., K.M.Q., C.R.B., K.K. and J.R.F. planned and conducted many of the biological studies. M.Y.G. and M.G.S. carried out the confocal microscopy studies on lymph node sections and polymer particles, respectively. T.H. and R.C. developed the plasmids to express the HIV Gag-coil fusion protein. R.L., M.P., R.P. and T.E. devised the coil-coil strategy. A.P.E.-K., T.E., K.D.F., L.W.S. and R.A.S. are principal investigators who advised the studies.
Corresponding author
Ethics declarations
Competing interests
G.M.L., R.L., K.D.F., L.W.S. and R.A.S. are listed as inventors on patents describing polymer-based vaccines. K.D.F. and L.W.S. are scientific founders and equity holders in PsiOxus Therapeutics, Ltd. (Oxford, UK). G.M.L. and J.R.F. are scientific founders and equity holders in Avidea Technologies, Inc., which is developing polymer-based technologies for immunotherapeutic applications (Baltimore, Maryland, USA).
Integrated supplementary information
Supplementary Figure 1 Synthesis of polymer-TLR-7/8a conjugates (Poly-7/8a).
(a) Chemical structures of imidazoquinoline-based TLR-7/8a. Polymer reactive analogs of the commercially available TLR-7/8a, R848, were produced by replacing the isopropanol group with reactive linkers that are indicated by the shaded boxes overlaying the structures of SM 7/8a, SM 7/8a-alkane and SM 7/8a-PEG. Note that the alkane and PEG linkers are of comparable length but different composition (hydrophobic vs. hydrophilic). The terminal amine on each of the linkers permitted facile attachment to amine reactive polymer precursors. (b) Poly-7/8a were generated by reacting nucleophilic TLR-7/8a (e.g., SM 7/8a) with HPMA-based copolymers in a one step reaction, resulting in a stable amide bond between the TLR-7/8a and the polymer backbone. Note that the brackets represent repeating units of each monomer, with the subscripts, x and y, representing the percentage composition (mol%) of each monomer. Poly = polymer; SM = small molecule; HPMA = N-(2-hydroxypropyl)methacrylamide; MA = methacrylamide; Ahx = aminohexanoic acid; PEG = Polyethylene glycol; TT = 2-Thiazolidine-2-thione.
Supplementary Figure 2 Combinatorial synthesis of Poly-7/8a.
(a) Structures of Imidazoquinoline-based TLR-7/8a used in the generation of combinatorial libraries of Poly-7/8a. In addition to SM 7/8a described previously, a ~ 20-fold more potent TLR-7/8a with a xylene linker was prepared and is referred to as SM 20x7/8a. The potency of the two TLR-7/8a were determined in vitro using HEK293 hTLR7 reporter cells. Absorbance at 620 nm in this experiment is proportional to TLR-7 activity. Note that acetylated versions of the TLR-7/8a were used in these in vitro assays as this best represents the physicochemical characteristics of the compounds when they are attached to the polymers. (b) A combinatorial library of Poly-7/8a was generated by attaching 2 unique TLR-7/8a (SM 7/8a or SM 20x7/8a) to reactive HPMA-based copolymers at different densities (~ 2, 4, 8 mol %) using short, alkane or PEG linkers. By reacting 2 unique TLR-7/8a at 3 different densities with 3 different linkers, 18 unique products can be generated, as illustrated (c). Note that this cartoon representation is for illustrative purposes; not all Poly-7/8a represented in this schematic were evaluated in this study, nor does this schematic represent all the materials described herein.
Supplementary Figure 3 Screening a combinatorial library of Poly-7/8a in vivo.
Combinatorial library of Poly-7/8a with varying TLR-7/8a density and linker group composition. (b) Cartoon schematic of a combinatorial library of Poly-7/8a. (c) Poly-7/8a normalized for TLR-7/8a dose (12.5 nmol) were subcutaneously administered into both hind footpads of C57BL/6 mice. After 24 h, draining lymph nodes (n = 4) were harvested and processed to generate a cell suspension that was cultured for 8 h and then evaluated for IP-10 production by ELISA. Data are reported as mean; statistical significance is reported relative to naïve (ANOVA with Bonferroni correction); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01.
Supplementary Figure 4 Increasing agonist density is associated with particle formation and TLR-7 dependent lymph node cytokine production.
(a) Properties of Poly-7/8a and controls. (b) Negative control polymers were generated using 2-aminopyridine (AP) to account for the contribution of the aromatic amine present on the Imidazoquinoline-based TLR-7/8a. AP was attached to polymers using a PEG or amphiphilic (AMPH) spacer to generate polymer coils and polymer particles, respectively. (c, d) Adjuvants were administered subcutaneously and after 24 h lymph nodes draining the site of immunization were harvested to create cell suspensions that were cultured for 8 h and then evaluated for (c) IFNα or (d) IFNγ by ELISA. (e, f) PP-7/8a (PEG, 10 mol% 7/8a) was administered subcutaneously to wild type (WT) or knockout mice and cytokines were evaluated from lymph node cell suspensions. All data are reported as mean ± SEM; except where indicated, statistical significance is relative to all other groups (ANOVA with Bonferroni correction, n = 4); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01
Supplementary Figure 5 In vivo tracking of dye-labeled Poly-7/8a.
(a) Properties of fluorescent dye-labeled materials. (b) Example gating tree. (c) Gates designating adjuvant positive cells (AF488+) were set relative to naïve. (d) Percent adjuvant uptake by the major CD11c+ DC subsets. (e-g) Evaluation of polymer controls and polymer particles with different densities of TLR-7/8a (3 and 10 mol% 7/8a) reveals that pharmacokinetics and uptake by APCs is primarily dependent on the morphology of the carrier (i.e. submicron particle) and is independent of the attached agonist. All data are reported as mean ± SEM. DC = dendritic cell; pDC = plasmacytoid dendritic cell; Mac = Macrophage; Mon = monocyte.
Supplementary Figure 6 Characterization of DC populations in draining lymph nodes and spleen.
AF488-labeled materials normalized for dose of TLR-7/8a (62.5 nmol) were unilaterally administered subcutaneously into the hind footpad of C57BL/6 mice. (a-c) At serial timepoints thereafter, lymph nodes (n = 3) or spleen (n = 1) were isolated and enzyme-digested to create cell suspensions that were stained and evaluated by flow cytometry. (a) Magnitude of DCs and (b) expression of the costimulatory molecule CD80 were evaluated by flow cytometry. (c) DC populations in the spleen were evaluated for costimulatory molecule expression (CD80 MFI) at serial timepoints. Note that the small molecule TLR-7/8a (SM 7/8a) leads to transient activation of the major DC subsets and B cells in both spleen and lymph nodes, whereas PP-7/8a leads to persistent activation of CD8- B220- DC (monocyte-derived DCs, skin-derived DCs and CD8- resident DCs) and CD8+ DC. Serum (n = 3) was evaluated for the presence of (h) IL-12p40 at serial timepoints. All data are reported as mean ± SEM; except where indicated, significance is relative to all other groups (ANOVA with Bonferroni correction); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01.
Supplementary Figure 7 Orientation of the TLR-7/8a attached to the polymer carrier influences the timing of onset and magnitude of lymph node cytokine production.
Poly-7/8a were prepared with TLR-7/8a attached to the polymer carrier with either the C4-amine exposed (1) (PP-20x7/8a) or blocked (2) (PP-R20x7/8a). (b, c) Poly-7/8a with two different orientations of TLR-7/8a were administered subcutaneously into the hind footpads of C57BL/6 mice and lymph nodes (n = 4) were isolated at serial timepoints thereafter and cultured overnight. Supernatant from the ex vivo lymph node cell suspensions (n = 4) were evaluated for (b) IL-12 and (c) IP-10 by ELISA. Note that blocking the C4-amine delays onset and leads to lower magnitude of cytokine production. All data are reported as mean ± SEM; significance is relative to all other groups (ANOVA with Bonferroni correction); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01.
Supplementary Figure 8 Anti-OVA antibody responses.
(a-d) Poly-7/8a, SM 7/8a or a control were formulated with 50 μg of OVA in PBS and given subcutaneously to C57BL/6 mice (n = 5) at days 0 and 14. Serum was collected from vaccinated mice at day 28 and evaluated for anti-OVA IgG1 and IgG2c antibodies. Doses of adjuvant and polymer are provided in the accompanying tables.
Supplementary Figure 9 Particle-forming Poly-7/8a induces locally restricted Th1-polarizing cytokines.
(a, b) R848 (62.5 nmol), PP-7/8a (62.5 nmol) or CpG ODN 1826 (3.1 nmol) were administered subcutaneously into the footpad of mice. Cytokine bead array was used to quantify cytokines present (a) in the serum (n = 5) at 6 h (peak), or (b) from draining lymph nodes (n = 4) at 24 h. All data are reported as mean ± SEM; except where indicated, statistical significance is relative to both OVA alone and OVA + R848 (ANOVA with Bonferroni correction); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01.
Supplementary Figure 10 Polymer conjugates of TLR-2/6 and TLR-4 agonists.
Structures of polymer conjugates of TLR-2/6 (Pam2Cys) and TLR-4 (Pyrimidoindole) agonists are shown above. Both agonists were linked to the polymer backbones through hydrophilic PEG spacers at > 5 mol % agonist density to induce polymer particle (PP) formation in aqueous conditions. Synthesis and characterization of PP-Pam2Cys and PP-PI conjugates is provided in the Supplementary Materials and Methods.
Supplementary Figure 11 Local and systemic innate immune activation and morbidity by particulate and unconjugated TLRa.
(a-d) TLR-2/6 agonists (PP-Pam2Cys and unconjugated Pam2Cys, 20 nmol), heterocyclic TLR-4 agonists (PP-PI and PI, 20 nmol), lipid-based TLR-4 agonists (50 μg Alum / MPL 5 μg or MPL alone (5 μg, ~3 nmol)), TLR-7/8a (PP-7/8a and R848, 12.5 nmol), TLR-9a (CpG/polyplex and CpG alone, 3 nmol), and controls were delivered subcutaneously into both hind footpads of C57BL/6 mice. Draining lymph nodes were harvested at day 4 (early peak for local activity) and were evaluated for (a) total CD11c+ DCs per lymph node (n = 3) and (b) IL-12p40 production (n = 8). (c) Serum (n = 5) was collected at 4 h (peak for systemic activity) post-immunization and was evaluated for IL-12p40 by ELISA. (d) Percent body weight change (n = 5) at peak (24 h) following subcutaneous administration of different vaccine adjuvants. (e) Meta-analysis of 4 independent studies (n = 43 groups) showing the relationship between systemic IL-12 production and body weight change (relative to time = 0) for mice immunized with either particle carriers of TLRa, unconjugated (free) TLRa or controls. PP = polymer particle; PI = pyrimidoindole; MPL = Monophosphoryl Lipid A; Alum = Aluminum hydroxide; CpG/Polyplex = Poly(Lysine).HCl complexed to CpG ODN 1826 at 20:1 N:P. See Supplementary Materials and Methods for chemical synthesis and formulation of the different TLRa. All data are representative of two or more independent experiments that included multiple time points. Data on linear scale are reported as mean ± SEM and data on log scale are reported as geometric mean ± 95% CI; statistical significance is shown for specific comparisons and for adjuvant formulations relative to naïve and particle controls (ANOVA with Bonferroni correction); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01.
Supplementary Figure 12 Particle-forming Poly-7/8a elicit protective CD8 T cell responses.
(a-c) Poly-7/8a, SM 7/8a and polymer controls admixed with 50 μg of OVA in 50 μL of PBS were administered subcutaneously into the hind footpad of C57BL/6 mice (n = 6) at days 0 and 14. Three different small molecule TLR-7/8a were evaluated in this experiment: either commercially available R848, SM 7/8a, or SM 20x7/8a. Poly-7/8a were evaluated for either dose, comparing PP-7/8a at 12.5 and 62.5 nmol, or potency of the agonist attached, comparing PP-7/8a with PP-20x7/8a. (b) The proportion of tetramer+ positive CD8 T responses was evaluated from whole blood at day 24. (c) Mice (n = 6) were challenged intravenously at day 28 with Listeria monocytogenes expressing full-length OVA Albumin (LM-OVA) and bacterial burdens in spleen was evaluated at day 31. Note that protection (inverse of bacterial burden) is proportional to the tetramer+ response. All data are reported as mean ± SEM; except where indicated, statistical significance is relative to both OVA alone and OVA + R848 (ANOVA with Bonferroni correction); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01.
Supplementary Figure 13 Particle forming Poly-7/8a elicit Th1 cells that mediate protection against Leishmania major.
(a-f) C57BL/6 mice received subcutaneous immunizations of 20 μg of MML protein from L major either alone or admixed with an adjuvant on days 0, 21 and 42. (a) Splenocytes were isolated on either (b) day 56 (n = 4) or (c, d) day 70 (n = 5) and stimulated in vitro with a peptide pool derived from MML. Antigen-specific CD4 T cells were evaluated for their capacity to produce Th1 characteristic cytokines (IFNγ, IL-2 or TNFα); (b) and (c) report total cytokine producing CD4 T cells (magnitude), whereas (d) reports the frequency of CD4 T cells producing combinations of IFNγ, IL-2 and TNFα (quality). (e) Mice (n = 6) were challenged intradermally in both ears with L major at day 70. Ear lesion diameters were measured up to 12 weeks after challenge. All data are reported as mean ± SEM; except where indicated, statistical significance is relative to naïve, MML alone and SM 7/8a (ANOVA with Bonferroni correction); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01.
Supplementary Figure 14 Temperature-responsive particle-forming Poly-7/8a (TRPP-7/8a) induce protective CD8 T cell responses.
(a) First-generation TRPP-7/8a are N-Isopropylacrylamide (NIPAM)-based copolymers. Note that the TLR-7/8a (7/8a or 20x7/8a) or a control ligand (AP) were attached to the NIPAM-based copolymers using a similar reaction scheme as described in supplementary figure 1 (see materials and methods). (b) A series of TRPP-7/8a were produced with increasing densities of either SM 7/8a, SM 20x7/8a or the control, AP-AMPH. Note that increasing densities of the hydrophobic ligands attached to the polymers leads to decreasing transition temperatures, the temperature at which particle formation occurs in aqueous solution. (c, d) TRPP-7/8a and controls were evaluated in a vaccination and challenge model using OVA. C57BL/6 mice (n = 5) received 50 μg of OVA either alone or admixed with adjuvant that was administered subcutaneously in 50 μL of PBS at days 0 and 14. (c) At day 24, the proportion of tetramer+ CD8 T cells was evaluated from whole blood. (d) The capacity of the tetramer+ CD8 T cells to mediate protection was assessed by challenging the mice intravenously at day 28 with LM-OVA. Bacterial burdens were assessed in the spleen at day 31.(e, f, g) Serum was collected from vaccinated mice at day 28 and evaluated for (e) anti-OVA IgG1 and (f) IgG2c antibodies as well as (g) the ratio of the two isotype titers (geometric mean). Data are reported as mean ± SEM; statistical significance is relative to OVA alone (ANOVA with Bonferroni correction); ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01.
Supplementary Figure 15 Self-assembling temperature-responsive vaccine particle.
(a) Schematic of a second-generation di-block copolymer-based TRPP-7/8a used for attachment of TLR-7/8a and a coil peptide. The hydrophilic block consists of HPMA and propargyl(methacrylamide) (PgMA). The acetylene group on PgMA allowed for the attachment of ligands that are modified with azide groups. The hydrophobic block is comprised of poly(diethylene glycol(methacrylate)) homopolymer that allows for the transition temperature to be independent of the attached ligands and contains a biodegradable ester group. Ligands (TLR-7/8a, peptide or fluorophore) were attached to the diblock copolymer through copper-catalyzed 1,3-dipolar cycloaddition. (b) Summary of TRPP-7/8a and TRPP controls. (c) Amino acid sequence of the HIV Gag p41 coil fusion protein; note that the coil domain (KSK) on the protein is complementary to the ESE coil peptide attached to the polymers. (d) Schematic representation of the anti-parallel coil-coil interactions that occur between the ESE and KSK coil peptides.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 and Supplementary Methods (PDF 3505 kb)
Rights and permissions
About this article
Cite this article
Lynn, G., Laga, R., Darrah, P. et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol 33, 1201–1210 (2015). https://doi.org/10.1038/nbt.3371
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3371
This article is cited by
-
Nanostructures for prevention, diagnosis, and treatment of viral respiratory infections: from influenza virus to SARS-CoV-2 variants
Journal of Nanobiotechnology (2023)
-
Engineering nanomaterial physical characteristics for cancer immunotherapy
Nature Reviews Bioengineering (2023)
-
A nanoadjuvant that dynamically coordinates innate immune stimuli activation enhances cancer immunotherapy and reduces immune cell exhaustion
Nature Nanotechnology (2023)
-
A Redox-responsive Prodrug Nanogel of TLR7/8 Agonist for Improved Cancer Immunotherapy
Chinese Journal of Polymer Science (2023)
-
Pattern recognition receptors and their nano-adjuvants for cancer immunotherapy
Journal of Pharmaceutical Investigation (2023)