Abstract
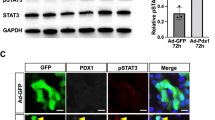
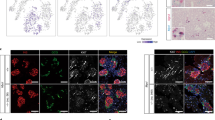
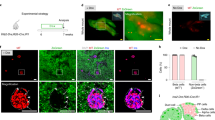
Reprogramming of pancreatic exocrine cells into cells resembling beta cells may provide a strategy for treating diabetes. Here we show that transient administration of epidermal growth factor and ciliary neurotrophic factor to adult mice with chronic hyperglycemia efficiently stimulates the conversion of terminally differentiated acinar cells to beta-like cells. Newly generated beta-like cells are epigenetically reprogrammed, functional and glucose responsive, and they reinstate normal glycemic control for up to 248 d. The regenerative process depends on Stat3 signaling and requires a threshold number of Neurogenin 3 (Ngn3)-expressing acinar cells. In contrast to previous work demonstrating in vivo conversion of acinar cells to beta-like cells by viral delivery of exogenous transcription factors, our approach achieves acinar-to-beta-cell reprogramming through transient cytokine exposure rather than genetic modification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brockes, J.P. & Kumar, A. Comparative aspects of animal regeneration. Annu. Rev. Cell Dev. Biol. 24, 525–549 (2008).
Slack, J.M. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat. Rev. Mol. Cell Biol. 8, 369–378 (2007).
Baddour, J.A., Sousounis, K. & Tsonis, P.A. Organ repair and regeneration: an overview. Birth Defects Res. C Embryo Today 96, 1–29 (2012).
Alvarado, A.S. & Tsonis, P.A. Bridging the regeneration gap: genetic insights from diverse animal models. Nat. Rev. Genet. 7, 873–884 (2006).
Dor, Y., Brown, J., Martinez, O.I. & Melton, D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004).
Nir, T., Melton, D.A. & Dor, Y. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 117, 2553–2561 (2007).
Xu, X. et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 (2008).
Solar, M. et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell 17, 849–860 (2009).
Kopinke, D. et al. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development 138, 431–441 (2011).
Kopinke, D. & Murtaugh, L.C. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev. Biol. 10, 38 (2010).
Furuyama, K. et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 43, 34–41 (2011).
Kopp, J.L. et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138, 653–665 (2011).
Thorel, F. et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464, 1149–1154 (2010).
Rooman, I. & Bouwens, L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia 47, 259–265 (2004).
Desai, B.M. et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J. Clin. Invest. 117, 971–977 (2007).
Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J. & Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455, 627–632 (2008).
Rooman, I. et al. Expression of the Notch signaling pathway and effect on exocrine cell proliferation in adult rat pancreas. Am. J. Pathol. 169, 1206–1214 (2006).
Rooman, I., Heremans, Y., Heimberg, H. & Bouwens, L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia 43, 907–914 (2000).
Means, A.L. et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132, 3767–3776 (2005).
Lardon, J. et al. Plasticity in the adult rat pancreas: transdifferentiation of exocrine to hepatocyte-like cells in primary culture. Hepatology 39, 1499–1507 (2004).
Baeyens, L. et al. Notch signaling as gatekeeper of rat acinar-to-beta-cell conversion in vitro. Gastroenterology 136, 1750–1760 (2009).
Baeyens, L. et al. Ngn3 expression during postnatal in vitro beta cell neogenesis induced by the JAK/STAT pathway. Cell Death Differ. 13, 1892–1899 (2006).
Baeyens, L. et al. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 48, 49–57 (2005).
Minami, K. et al. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc. Natl. Acad. Sci. USA 102, 15116–15121 (2005).
Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 50, 537–546 (2001).
Pan, F.C. et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 140, 751–764 (2013).
Mellitzer, G. et al. Pancreatic islet progenitor cells in Neurogenin 3-yellow fluorescent protein knock-add-on mice. Mol. Endocrinol. 18, 2765–2776 (2004).
Miettinen, P., Ormio, P., Hakonen, E., Banerjee, M. & Otonkoski, T. EGF receptor in pancreatic beta-cell mass regulation. Biochem. Soc. Trans. 36, 280–285 (2008).
Miettinen, P.J. et al. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development 127, 2617–2627 (2000).
Wadt, K.A. et al. Ciliary neurotrophic factor potentiates the beta-cell inhibitory effect of IL-1beta in rat pancreatic islets associated with increased nitric oxide synthesis and increased expression of inducible nitric oxide synthase. Diabetes 47, 1602–1608 (1998).
Ip, N.Y. et al. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell 69, 1121–1132 (1992).
Murakami, M. et al. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260, 1808–1810 (1993).
Lambert, P.D. et al. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc. Natl. Acad. Sci. USA 98, 4652–4657 (2001).
Gloaguen, I. et al. Ciliary neurotrophic factor corrects obesity and diabetes associated with leptin deficiency and resistance. Proc. Natl. Acad. Sci. USA 94, 6456–6461 (1997).
Watt, M.J. et al. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat. Med. 12, 541–548 (2006).
Sleeman, M.W. et al. Ciliary neurotrophic factor improves diabetic parameters and hepatic steatosis and increases basal metabolic rate in db/db mice. Proc. Natl. Acad. Sci. USA 100, 14297–14302 (2003).
Rezende, L.F., Santos, G.J., Santos-Silva, J.C., Carneiro, E.M. & Boschero, A.C. Ciliary neurotrophic factor (CNTF) protects non-obese Swiss mice against type 2 diabetes by increasing beta cell mass and reducing insulin clearance. Diabetologia 55, 1495–1504 (2012).
Rezende, L.F., Vieira, A.S., Negro, A., Langone, F. & Boschero, A.C. Ciliary neurotrophic factor (CNTF) signals through STAT3-SOCS3 pathway and protects rat pancreatic islets from cytokine-induced apoptosis. Cytokine 46, 65–71 (2009).
Kamakura, S. et al. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat. Cell Biol. 6, 547–554 (2004).
Gradwohl, G., Dierich, A., LeMeur, M. & Guillemot, F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97, 1607–1611 (2000).
Apelqvist, A. et al. Notch signalling controls pancreatic cell differentiation. Nature 400, 877–881 (1999).
Wang, S. et al. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc. Natl. Acad. Sci. USA 106, 9715–9720 (2009).
Yu, L. et al. BMP signaling induces digit regeneration in neonatal mice. Development 137, 551–559 (2010).
Han, M., Yang, X., Farrington, J.E. & Muneoka, K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development 130, 5123–5132 (2003).
Rinkevich, Y., Lindau, P., Ueno, H., Longaker, M.T. & Weissman, I.L. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476, 409–413 (2011).
Lehoczky, J.A., Robert, B. & Tabin, C.J. Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc. Natl. Acad. Sci. USA 108, 20609–20614 (2011).
Odelberg, S.J., Kollhoff, A. & Keating, M.T. Dedifferentiation of mammalian myotubes induced by msx1. Cell 103, 1099–1109 (2000).
Mellitzer, G. et al. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J. 25, 1344–1352 (2006).
Schwitzgebel, V.M. et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 127, 3533–3542 (2000).
Wright, C.V., Schnegelsberg, P. & De Robertis, E.M. XlHbox 8: a novel Xenopus homeo protein restricted to a narrow band of endoderm. Development 105, 787–794 (1989).
Acknowledgements
Special thanks to V. Laurysens, A. Demarré, E. Quartier, J. De Jonge for technical advice and assistance, D. Pipeleers for logistic support and G. Bin for the Stat3lox/lox mouse strain. We thank J. Kornak for support on biostatistics. L.Ba. is a postdoctoral fellow of the Research Foundation – Flanders (FWO). Financial support was obtained from the Research Foundation – Flanders (FWO) (H.H., L.Ba. and L.Bo.), the Juvenile Diabetes Research Foundation (H.H. and L.Bo.), DON Foundation (www.sdon.nl) (H.H.), the European Foundation for the Study of Diabetes (EFSD) (L.Ba., P.B. and L.Bo.), the European Union Sixth (#LSHB-CT-2005-512145), Seventh (HEALTH-F5-2009-241883) Framework Program (H.H. and L.Bo.), Diabetesfonds Nederland (H.H.), NIH U19 DK 042502 and U01 DK 089570 (C.V.E.W. and F.C.P.), P30 DK063720 and U01 DK089541 (M.S.G.).
Author information
Authors and Affiliations
Contributions
Design: L.Ba., H.H.; execution of experiments: L.Ba., M.L., G.L., S.D.G., R.S., C.N., D.W.S.; analyses: L.Ba., M.L.; transgenic mouse strain generation: F.C.P., C.V.E.W., Y.D., D.A.S., G.G., J.F., G.G.; cell sorting: L.Ba., G.S.; interpretation of results: L.Ba., M.L., P.B., U.A., M.S.G., M.V.d.C., H.H.; writing: L.Ba., M.S.G., H.H.; critical reading: L.Ba., P.B., C.V.E.W., Y.D., J.F., G.G., L.Bo., M.V.d.C., M.S.G., H.H.; project management: M.S.G., L.Bo., H.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 24854 kb)
Rights and permissions
About this article
Cite this article
Baeyens, L., Lemper, M., Leuckx, G. et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol 32, 76–83 (2014). https://doi.org/10.1038/nbt.2747
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2747
This article is cited by
-
Stem cells differentiation into insulin-producing cells (IPCs): recent advances and current challenges
Stem Cell Research & Therapy (2022)
-
Mesenchymal stem cells promote pancreatic β-cell regeneration through downregulation of FoxO1 pathway
Stem Cell Research & Therapy (2020)
-
STAT3 dictates β-cell apoptosis by modulating PTEN in streptozocin-induced hyperglycemia
Cell Death & Differentiation (2020)
-
Adult human pancreatic acinar cells dedifferentiate into an embryonic progenitor-like state in 3D suspension culture
Scientific Reports (2019)
-
Organoids from the Human Fetal and Adult Pancreas
Current Diabetes Reports (2019)