Abstract
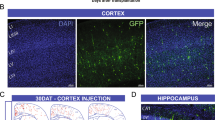
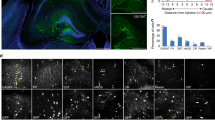
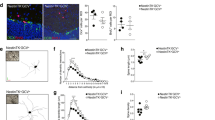
Dysfunction of basal forebrain cholinergic neurons (BFCNs) and γ-aminobutyric acid (GABA) interneurons, derived from medial ganglionic eminence (MGE), is implicated in disorders of learning and memory. Here we present a method for differentiating human embryonic stem cells (hESCs) to a nearly uniform population of NKX2.1+ MGE-like progenitor cells. After transplantation into the hippocampus of mice in which BFCNs and some GABA neurons in the medial septum had been destroyed by mu P75-saporin, human MGE-like progenitors, but not ventral spinal progenitors, produced BFCNs that synaptically connected with endogenous neurons, whereas both progenitors generated similar populations of GABA neurons. Mice transplanted with MGE-like but not spinal progenitors showed improvements in learning and memory deficits. These results suggest that progeny of the MGE-like progenitors, particularly BFCNs, contributed to learning and memory. Our findings support the prospect of using human stem cell–derived MGE-like progenitors in developing therapies for neurological disorders of learning and memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sussel, L., Marin, O., Kimura, S. & Rubenstein, J.L. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126, 3359–3370 (1999).
Rubenstein, J.L., Shimamura, K., Martinez, S. & Puelles, L. Regionalization of the prosencephalic neural plate. Annu. Rev. Neurosci. 21, 445–477 (1998).
Wilson, S.W. & Rubenstein, J.L. Induction and dorsoventral patterning of the telencephalon. Neuron 28, 641–651 (2000).
Zhao, Y. et al. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc. Natl. Acad. Sci. USA 100, 9005–9010 (2003).
Campbell, K. Dorsal-ventral patterning in the mammalian telencephalon. Curr. Opin. Neurobiol. 13, 50–56 (2003).
Woolf, N.J. Cholinergic systems in mammalian brain and spinal cord. Prog. Neurobiol. 37, 475–524 (1991).
Oliveira, A.A. Jr. & Hodges, H.M. Alzheimer's disease and neural transplantation as prospective cell therapy. Curr. Alzheimer Res. 2, 79–95 (2005).
Whitehouse, P.J. et al. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239 (1982).
Pang, K.C., Jiao, X., Sinha, S., Beck, K.D. & Servatius, R.J. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: effects on proactive interference. Hippocampus. 21, 835–846 (2011).
Dwyer, T.A., Servatius, R.J. & Pang, K.C. Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J. Neurosci. 27, 299–303 (2007).
Zhang, S.C. Neural subtype specification from embryonic stem cells. Brain Pathol. 16, 132–142 (2006).
Yamanaka, S. A fresh look at iPS cells. Cell 137, 13–17 (2009).
Li, X.J. et al. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 23, 215–221 (2005).
Pankratz, M.T. et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells 25, 1511–1520 (2007).
Li, X.J. et al. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development 136, 4055–4063 (2009).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Ma, L. et al. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 10, 455–464 (2012).
Lee, S.H., Lumelsky, N., Studer, L., Auerbach, J.M. & McKay, R.D. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 18, 675–679 (2000).
Xi, J. et al. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 30, 1655–1663 (2012).
Kirkeby, A. et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 1, 703–714 (2012).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547–551 (2011).
Singh Roy, N. et al. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp. Neurol. 196, 224–234 (2005).
Yang, D., Zhang, Z.J., Oldenburg, M., Ayala, M. & Zhang, S.C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 26, 55–63 (2008).
Roy, N.S. et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 12, 1259–1268 (2006).
Cooper, O. et al. Differentiation of human ES and Parkinson's disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol. Cell Neurosci. 45, 258–266 (2010).
Hargus, G. et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl. Acad. Sci. USA 107, 15921–15926 (2010).
Zhang, S.C., Wernig, M., Duncan, I.D., Brustle, O. & Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19, 1129–1133 (2001).
Flames, N. et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 27, 9682–9695 (2007).
Manabe, T. et al. L3/Lhx8 is involved in the determination of cholinergic or GABAergic cell fate. J. Neurochem. 94, 723–730 (2005).
Krencik, R., Weick, J.P., Liu, Y., Zhang, Z.J. & Zhang, S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 29, 528–534 (2011).
Reilly, J.O., Karavanova, I.D., Williams, K.P., Mahanthappa, N.K. & Allendoerfer, K.L. Cooperative effects of Sonic Hedgehog and NGF on basal forebrain cholinergic neurons. Mol. Cell Neurosci. 19, 88–96 (2002).
Cassel, J.C. et al. Grafts of fetal septal cells after cholinergic immunotoxic denervation of the hippocampus: a functional dissociation between dorsal and ventral implantation sites. Neuroscience 113, 871–882 (2002).
Leanza, G., Martinez-Serrano, A. & Bjorklund, A. Amelioration of spatial navigation and short-term memory deficits by grafts of foetal basal forebrain tissue placed into the hippocampus and cortex of rats with selective cholinergic lesions. Eur. J. Neurosci. 10, 2353–2370 (1998).
Berger-Sweeney, J. et al. Selective immunolesions of cholinergic neurons in mice: effects on neuroanatomy, neurochemistry, and behavior. J. Neurosci. 21, 8164–8173 (2001).
Walsh, T.J., Herzog, C.D., Gandhi, C., Stackman, R.W. & Wiley, R.G. Injection of IgG 192-saporin into the medial septum produces cholinergic hypofunction and dose-dependent working memory deficits. Brain Res. 726, 69–79 (1996).
Jinno, S. Regional and laminar differences in antigen profiles and spatial distributions of astrocytes in the mouse hippocampus, with reference to aging. Neuroscience 180, 41–52 (2011).
Francis, P.T. Glutamatergic systems in Alzheimer's disease. Int. J. Geriatr. Psychiatry 18, S15–S21 (2003).
Weick, J.P., Liu, Y. & Zhang, S.C. Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network. Proc. Natl. Acad. Sci. USA 108, 20189–20194 (2011).
Weick, J.P. et al. Functional control of transplantable human ESC-derived neurons via optogenetic targeting. Stem Cells 28, 2008–2016 (2010).
Danjo, T. et al. Subregional specification of embryonic stem cell–derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J. Neurosci. 31, 1919–1933 (2011).
Bissonnette, C.J. et al. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells 29, 802–811 (2011).
Pankratz, M.T. et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells 25, 1511–1520 (2007).
Martinez-Cerdeno, V. et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell 6, 238–250 (2010).
Gage, F.H., Bjorklund, A., Stenevi, U., Dunnett, S.B. & Kelly, P.A. Intrahippocampal septal grafts ameliorate learning impairments in aged rats. Science 225, 533–536 (1984).
Emborg, M.E. et al. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 17, 383–395 (2008).
Gage, F.H. & Bjorklund, A. Trophic and growth-regulating mechanisms in the central nervous system monitored by intracerebral neural transplants. Ciba Found. Symp. 126, 143–159 (1987).
Lecourtier, L. et al. Septohippocampal pathways contribute to system consolidation of a spatial memory: sequential implication of gabaergic and cholinergic neurons. Hippocampus 21, 1277–1289 (2011).
Aubry, L. et al. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc. Natl. Acad. Sci. USA 105, 16707–16712 (2008).
Li, X.J. et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells 26, 886–893 (2008).
Hu, B.Y. & Zhang, S.C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 4, 1295–1304 (2009).
Du, Z.W., Hu, B.Y., Ayala, M., Sauer, B. & Zhang, S.C. Cre recombination-mediated cassette exchange for building versatile transgenic human embryonic stem cells lines. Stem Cells 27, 1032–1041 (2009).
Johnson, M.A., Weick, J.P., Pearce, R.A. & Zhang, S.C. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J. Neurosci. 27, 3069–3077 (2007).
LaVaute, T.M. et al. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells 27, 1741–1749 (2009).
Peterson, D.A. Quantitative histology using confocal microscopy: implementation of unbiased stereology procedures. Methods 18, 493–507 (1999).
Acknowledgements
We thank M.E. Andrzejewski and H. Mitchell for help in analyzing animal behavioral data. This study was supported in part by the US National Institute of Neurological Disorders and Stroke (NS045926) and the National Institute of Child Health and Human Development (P30 HD03352).
Author information
Authors and Affiliations
Contributions
Y.L. and S.-C.Z. designed the experiments and wrote the manuscript. Y.L., J.P.W., H.L., R.K., X.Z., L.M., G.Z. and M.A. performed the experiments. Y.L., J.P.W., H.L., R.K., X.Z., L.M., M.A. and S.-C.Z. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–4 (PDF 5554 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Weick, J., Liu, H. et al. Medial ganglionic eminence–like cells derived from human embryonic stem cells correct learning and memory deficits. Nat Biotechnol 31, 440–447 (2013). https://doi.org/10.1038/nbt.2565
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2565
This article is cited by
-
Epigenetic regulation and factors that influence the effect of iPSCs-derived neural stem/progenitor cells (NS/PCs) in the treatment of spinal cord injury
Clinical Epigenetics (2024)
-
Genetic analysis of the X chromosome in people with Lewy body dementia nominates new risk loci
npj Parkinson's Disease (2024)
-
Potential use of iPSCs for disease modeling, drug screening, and cell-based therapy for Alzheimer’s disease
Cellular & Molecular Biology Letters (2023)
-
Miniature-swine iPSC-derived GABA progenitor cells function in a rat Parkinson’s disease model
Cell and Tissue Research (2023)
-
Pathophysiological Aspects and Therapeutic Armamentarium of Alzheimer’s Disease: Recent Trends and Future Development
Cellular and Molecular Neurobiology (2023)