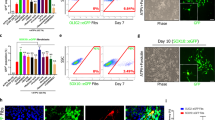
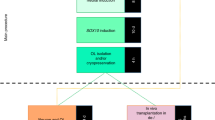
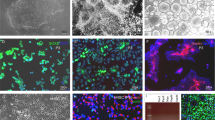
Abstract
Cell-based therapies for myelin disorders, such as multiple sclerosis and leukodystrophies, require technologies to generate functional oligodendrocyte progenitor cells. Here we describe direct conversion of mouse embryonic and lung fibroblasts to induced oligodendrocyte progenitor cells (iOPCs) using sets of either eight or three defined transcription factors. iOPCs exhibit a bipolar morphology and global gene expression profile consistent with bona fide OPCs. They can be expanded in vitro for at least five passages while retaining the ability to differentiate into multiprocessed oligodendrocytes. When transplanted to hypomyelinated mice, iOPCs are capable of ensheathing host axons and generating compact myelin. Lineage conversion of somatic cells to expandable iOPCs provides a strategy to study the molecular control of oligodendrocyte lineage identity and may facilitate neurological disease modeling and autologous remyelinating therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goldman, S.A., Nedergaard, M. & Windrem, M.S. Glial progenitor cell-based treatment and modeling of neurological disease. Science 338, 491–495 (2012).
Franklin, R.J. & Ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 (2008).
Windrem, M.S. et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell 2, 553–565 (2008).
Sim, F. et al. CD140a identifies a population of highly myelinogenic, migration-competent, and efficiently engrafting human oligodendrocyte progenitor cells. Nat. Biotechnol. (2011).
Najm, F.J. et al. Rapid and robust generation of functional oligodendrocyte progenitor cells from epiblast stem cells. Nat. Methods 8, 957–962 (2011).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Pang, Z.P. et al. Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 (2011).
Caiazzo, M. et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 (2011).
Pfisterer, U. et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA 108, 10343–10348 (2011).
Yoo, A.S. et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476, 228–231 (2011).
Kim, J. et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl. Acad. Sci. USA 108, 7838–7843 (2011).
Son, E.Y. et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9, 205–218 (2011).
Han, D.W. et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10, 465–472 (2012).
Thier, M. et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10, 473–479 (2012).
Lujan, E., Chanda, S., Ahlenius, H., Sudhof, T.C. & Wernig, M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl. Acad. Sci. USA 109, 2527–2532 (2012).
Cahoy, J.D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Liu, Z. et al. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev. Biol. 302, 683–693 (2007).
Zhang, X. et al. Induction of oligodendrocytes from adult human olfactory epithelial-derived progenitors by transcription factors. Stem Cells 23, 442–453 (2005).
Mallon, B.S., Shick, H.E., Kidd, G.J. & Macklin, W.B. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J. Neurosci. 22, 876–885 (2002).
Beard, C., Hochedlinger, K., Plath, K., Wutz, A. & Jaenisch, R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis 44, 23–28 (2006).
Bogler, O., Wren, D., Barnett, S.C., Land, H. & Noble, M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc. Natl. Acad. Sci. USA 87, 6368–6372 (1990).
Noble, M., Murray, K., Stroobant, P., Waterfield, M.D. & Riddle, P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333, 560–562 (1988).
Richardson, W.D., Pringle, N., Mosley, M.J., Westermark, B. & Dubois-Dalcq, M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell 53, 309–319 (1988).
Noll, E. & Miller, R.H. Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development 118, 563–573 (1993).
Rowitch, D.H. & Kriegstein, A.R. Developmental genetics of vertebrate glial-cell specification. Nature 468, 214–222 (2010).
Watkins, T.A., Emery, B., Mulinyawe, S. & Barres, B.A. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60, 555–569 (2008).
Crang, A.J., Gilson, J. & Blakemore, W.F. The demonstration by transplantation of the very restricted remyelinating potential of post-mitotic oligodendrocytes. J. Neurocytol. 27, 541–553 (1998).
Barres, B.A., Lazar, M.A. & Raff, M.C. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097–1108 (1994).
McLean, C.Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Chernoff, G.F. Shiverer: an autosomal recessive mutant mouse with myelin deficiency. J. Hered. 72, 128 (1981).
Gahwiler, B.H., Capogna, M., Debanne, D., McKinney, R.A. & Thompson, S.M. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 20, 471–477 (1997).
Bai, L. et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat. Neurosci. 15, 862–870 (2012).
Zawadzka, M. et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6, 578–590 (2010).
Mi, S. et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann. Neurol. 65, 304–315 (2009).
Yano, S. et al. An antigen retrieval method using an alkaline solution allows immunoelectron microscopic identification of secretory granules in conventional epoxy-embedded tissue sections. J. Histochem. Cytochem. 51, 199–204 (2003).
Denk, W. & Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2, e329 (2004).
Mikula, S., Binding, J. & Denk, W. Staining and embedding the whole mouse brain for electron microscopy. Nat. Methods 9, 1198–1201 (2012).
Acknowledgements
This research was supported by US National Institutes of Health (NIH) grants MH087877 (P.J.T. and R.H.M.), NS30800 (R.H.M.) and EY019880 (T.M.); NIH predoctoral training grants F31NS083354 (A.M.L.) and T32GM008056 (R.T.K.); Case Western Reserve University School of Medicine (P.J.T.); the New York Stem Cell Foundation (P.J.T.); the Mt. Sinai Health Care Foundation (P.J.T.); and the Cytometry & Imaging Microscopy, and the Gene Expression and Genotyping core facilities of the Case Comprehensive Cancer Center (P30CA043703). P.J.T. is a New York Stem Cell Foundation–Robertson Investigator. We are grateful to L. Cooperman, J. Wanta, M. Hitomi, J. Krasno, M. Karl, S. Fyffe-Maricich, S. Wu, M. Pendergast, O. Corradin, T. LaFramboise and P. Scacheri for technical assistance, I. Tsung for artwork, as well as N. Avishai and G. Kidd for 3D scanning electron microscopy imaging and data processing.
Author information
Authors and Affiliations
Contributions
F.J.N., A.M.L. and P.J.T. designed the reprogramming strategy and generated all iOPCs; F.J.N., A.M.L., R.T.K. and P.J.T. performed in vitro differentiation experiments; A.V.C., A.Z., F.J.N., A.M.L. and T.M. performed in vivo myelination experiments; A.Z., F.J.N., A.M.L., R.H.M. and P.J.T. performed slice culture myelination experiments; K.W., A.Z. and R.H.M. produced electron microscope images; F.J.N., A.M.L., D.C.F. and P.J.T. generated and analyzed gene expression data; F.J.N., A.M.L., R.H.M. and P.J.T. analyzed all of the data and wrote the paper. All authors edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
P.J.T., R.H.M. and F.J.N. have a pending patent application for this technology.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 7874 kb)
Supplementary Table 1
Transcription factors enriched in CNS lineages (used to generate Figure 1b) (XLS 21 kb)
Supplementary Table 2
GREAT analysis for Najm, Lager, et al. (2013) (XLS 186 kb)
Supplementary Movie 1
3-dimensional reconstruction of serial block-face scanning electron micrographs showing iOPC myelin in the dorsal column of the hypomyelinated shiverer spinal cord (MOV 30996 kb)
Rights and permissions
About this article
Cite this article
Najm, F., Lager, A., Zaremba, A. et al. Transcription factor–mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol 31, 426–433 (2013). https://doi.org/10.1038/nbt.2561
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2561
This article is cited by
-
Cell replacement therapy with stem cells in multiple sclerosis, a systematic review
Human Cell (2023)
-
Direct reprogramming of human fibroblasts into insulin-producing cells using transcription factors
Communications Biology (2023)
-
EGF signaling promotes the lineage conversion of astrocytes into oligodendrocytes
Molecular Medicine (2022)
-
The landscape of targets and lead molecules for remyelination
Nature Chemical Biology (2022)
-
OCT4-induced oligodendrocyte progenitor cells promote remyelination and ameliorate disease
npj Regenerative Medicine (2022)