Abstract
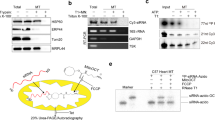
Synthetic small interfering RNAs (siRNAs) are an indispensable tool to investigate gene function in eukaryotic cells1,2 and may be used for therapeutic purposes to knock down genes implicated in disease3. Thus far, most synthetic siRNAs have been produced by chemical synthesis. Here we present a method to produce highly potent siRNAs in Escherichia coli. This method relies on ectopic expression of p19, an siRNA-binding protein found in a plant RNA virus4,5. When expressed in E. coli, p19 stabilizes an ∼21-nt siRNA-like species produced by bacterial RNase III. When mammalian cells are transfected by them, siRNAs that were generated in bacteria expressing p19 and a hairpin RNA encoding 200 or more nucleotides of a target gene reproducibly knock down target gene expression by ∼90% without immunogenicity or off-target effects. Because bacterially produced siRNAs contain multiple sequences against a target gene, they may be especially useful for suppressing polymorphic cellular or viral genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Gene Expression Omnibus
Sequence Read Archive
Referenced accessions
NCBI Reference Sequence
Change history
22 March 2013
In the version of this article initially published online, in Figure 4d, the label EGFPPFL vs NC siRNA has been corrected to read EGFPFL vs NC siRNA. The error has been corrected for the print, PDF and HTML versions of this article.
References
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Caplen, N.J., Parrish, S., Imani, F., Fire, A. & Morgan, R.A. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. USA 98, 9742–9747 (2001).
Rettig, G.R. & Behlke, M.A. Progress toward in vivo use of siRNAs-II. Mol. Ther. 20, 483–512 (2012).
Voinnet, O., Pinto, Y.M. & Baulcombe, D.C. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA 96, 14147–14152 (1999).
Silhavy, D. et al. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J. 21, 3070–3080 (2002).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Hamilton, A.J. & Baulcombe, D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286, 950–952 (1999).
Myers, J.W., Jones, J.T., Meyer, T. & Ferrell, J.E. Jr. Recombinant Dicer efficiently converts large dsRNAs into siRNAs suitable for gene silencing. Nat. Biotechnol. 21, 324–328 (2003).
Yang, D. et al. Short RNA duplexes produced by hydrolysis with Escherichia coli RNase III mediate effective RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 9942–9947 (2002).
Morlighem, J.E., Petit, C. & Tzertzinis, G. Determination of silencing potency of synthetic and RNase III-generated siRNA using a secreted luciferase assay. Biotechniques 42, 599–605 (2007).
Semizarov, D. et al. Specificity of short interfering RNA determined through gene expression signatures. Proc. Natl. Acad. Sci. USA 100, 6347–6352 (2003).
Vargason, J.M., Szittya, G., Burgyan, J. & Hall, T.M. Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115, 799–811 (2003).
Jin, J., Cid, M., Poole, C.B. & McReynolds, L.A. Protein mediated miRNA detection and siRNA enrichment using p19. Biotechniques 48, xvii–xxiii (2010).
Davis, M.G. & Huang, E.S. Transfer and expression of plasmids containing human cytomegalovirus immediate-early gene 1 promoter-enhancer sequences in eukaryotic and prokaryotic cells. Biotechnol. Appl. Biochem. 10, 6–12 (1988).
Chu, M., Desvoyes, B., Turina, M., Noad, R. & Scholthof, H.B. Genetic dissection of tomato bushy stunt virus p19-protein-mediated host-dependent symptom induction and systemic invasion. Virology 266, 79–87 (2000).
Knight, S.W. & Bass, B.L. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293, 2269–2271 (2001).
Timmons, L., Court, D.L. & Fire, A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103–112 (2001).
Babitzke, P., Granger, L., Olszewski, J. & Kushner, S.R. Analysis of mRNA decay and rRNA processing in Escherichia coli multiple mutants carrying a deletion in RNase III. J. Bacteriol. 175, 229–239 (1993).
Cummins, J.M. et al. The colorectal microRNAome. Proc. Natl. Acad. Sci. USA 103, 3687–3692 (2006).
Jackson, A.L. et al. Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing. RNA 12, 1197–1205 (2006).
Spankuch, B. et al. Cancer inhibition in nude mice after systemic application of U6 promoter-driven short hairpin RNAs against PLK1. J. Natl. Cancer Inst. 96, 862–872 (2004).
Lee, S.K. et al. Lentiviral delivery of short hairpin RNAs protects CD4 T cells from multiple clades and primary isolates of HIV. Blood 106, 818–826 (2005).
Sugiyama, R., Habu, Y., Ohnari, A., Miyano-Kurosaki, N. & Takaku, H. RNA interference targeted to the conserved dimerization initiation site (DIS) of HIV-1 restricts virus escape mutation. J. Biochem. 146, 481–489 (2009).
Jayaprakash, A.D., Jabado, O., Brown, B.D. & Sachidanandam, R. Identification and remediation of biases in the activity of RNA ligases in small-RNA deep sequencing. Nucleic Acids Res. 39, e141 (2011).
Weinberg, D.E., Nakanishi, K., Patel, D.J. & Bartel, D.P. The inside-out mechanism of Dicers from budding yeasts. Cell 146, 262–276 (2011).
Jackson, A.L. et al. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 21, 635–637 (2003).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Korb, M. et al. The Innate Immune Database (IIDB). BMC Immunol. 9, 7 (2008).
Tenllado, F., Martinez-Garcia, B., Vargas, M. & Diaz-Ruiz, J.R. Crude extracts of bacterially expressed dsRNA can be used to protect plants against virus infections. BMC Biotechnol. 3, 3 (2003).
Zhao, H.F. et al. High-throughput screening of effective siRNAs from RNAi libraries delivered via bacterial invasion. Nat. Methods 2, 967–973 (2005).
Xiang, S., Fruehauf, J. & Li, C.J. Short hairpin RNA-expressing bacteria elicit RNA interference in mammals. Nat. Biotechnol. 24, 697–702 (2006).
Nakanishi, K., Weinberg, D.E., Bartel, D.P. & Patel, D.J. Structure of yeast Argonaute with guide RNA. Nature 486, 368–374 (2012).
Princen, K., Hatse, S., Vermeire, K., De Clercq, E. & Schols, D. Establishment of a novel CCR5 and CXCR4 expressing CD4+ cell line which is highly sensitive to HIV and suitable for high-throughput evaluation of CCR5 and CXCR4 antagonists. Retrovirology 1, 2 (2004).
Dancz, C.E., Haraga, A., Portnoy, D.A. & Higgins, D.E. Inducible control of virulence gene expression in Listeria monocytogenes: temporal requirement of listeriolysin O during intracellular infection. J. Bacteriol. 184, 5935–5945 (2002).
Pall, G.S. & Hamilton, A.J. Improved northern blot method for enhanced detection of small RNA. Nat. Protoc. 3, 1077–1084 (2008).
Li, C. & Wong, W.H. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA 98, 31–36 (2001).
Acknowledgements
The authors thank J. Carrington (Donald Danforth Plant Science Center) for the p19 clone, C. Zhang and G. Ruvkun (Massachusetts General Hospital) for L4440 plasmid and HT115(DE3) strain, S. Kushner (University of Georgia) for SK7622 strain, D. Higgins (Harvard Medical School) for pLIV-1 plasmid, and S. Ranjbar (Boston Children's Hospital) for HIV strains and cell lines. We thank L.M. Mazzola, J.M. Bybee and D.B. Munafo from New England Biolabs for assistance with RNA deep sequencing and Z. Ansara for technical help. We thank A. Hochschild (Harvard Medical School) for suggestions and critical reading of the manuscript and Lieberman Lab members for technical assistance, helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health grant AI087431 (J.L.) and a GSK-IDI Alliance fellowship (L.H.).
Author information
Authors and Affiliations
Contributions
L.H. and J.L. designed the experiments with advice from J.J., L.M., and P.D. J.J. and L.M. prepared p19 beads and RNA deep sequencing libraries. P.D. constructed E. coli mutant strains. E.K. performed siRNA comparison and macrophage transfection experiments. L.H. performed all other experiments. L.H. and J.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
J.J. and L.M. are employees of New England Biolabs, a company that sells deep sequencing kits, p19 and other proteins for RNA and DNA research.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1–3 and 5–8 (PDF 1425 kb)
Supplementary Table 4
Significantly changed genes (XLSX 104 kb)
Rights and permissions
About this article
Cite this article
Huang, L., Jin, J., Deighan, P. et al. Efficient and specific gene knockdown by small interfering RNAs produced in bacteria. Nat Biotechnol 31, 350–356 (2013). https://doi.org/10.1038/nbt.2537
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2537
This article is cited by
-
A novel recombinant ORF7-siRNA delivered by flexible nano-liposomes inhibits varicella zoster virus infection
Cell & Bioscience (2023)
-
Topical application of dsRNA for plant virus control: a review
Tropical Plant Pathology (2022)
-
Advancing toward commercial application of RNA silencing-based strategies to protect plants from viral diseases
Journal of General Plant Pathology (2019)
-
Knocking down disease: a progress report on siRNA therapeutics
Nature Reviews Genetics (2015)
-
The effects of RNA interference targeting Bactrocera dorsalis ds-Bdrpl19 on the gene expression of rpl19 in non-target insects
Ecotoxicology (2015)