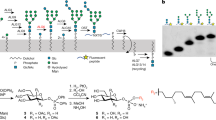
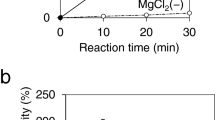
Abstract
Lysosomal storage diseases are treated with human lysosomal enzymes produced in mammalian cells. Such enzyme therapeutics contain relatively low levels of mannose-6-phosphate, which is required to target them to the lysosomes of patient cells. Here we describe a method for increasing mannose-6-phosphate modification of lysosomal enzymes produced in yeast. We identified a glycosidase from C. cellulans that 'uncaps' N-glycans modified by yeast-type mannose-Pi-6-mannose to generate mammalian-type N-glycans with a mannose-6-phosphate substitution. Determination of the crystal structure of this glycosidase provided insight into its substrate specificity. We used this uncapping enzyme together with α-mannosidase to produce in yeast a form of the Pompe disease enzyme α-glucosidase rich in mannose-6-phosphate. Compared with the currently used therapeutic version, this form of α-glucosidase was more efficiently taken up by fibroblasts from Pompe disease patients, and it more effectively reduced cardiac muscular glycogen storage in a mouse model of the disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Futerman, A.H. & van Meer, G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5, 554–565 (2004).
Desnick, R.J. & Schuchman, E.H. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat. Rev. Genet. 3, 954–966 (2002).
Pohl, S., Marschner, K., Storch, S. & Braulke, T. Glycosylation- and phosphorylation-dependent intracellular transport of lysosomal hydrolases. Biol. Chem. 390, 521–527 (2009).
Dahms, N.M., Lobel, P. & Kornfeld, S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J. Biol. Chem. 264, 12115–12118 (1989).
Hille-Rehfeld, A. Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. Biochim. Biophys. Acta 1241, 177–194 (1995).
Chiba, Y. et al. Production in yeast of α-galactosidase A, a lysosomal enzyme applicable to enzyme replacement therapy for Fabry disease. Glycobiology 12, 821–828 (2002).
Braulke, T., Gartung, C., Hasilik, A. & von Figura, K. Is movement of mannose 6-phosphate-specific receptor triggered by binding of lysosomal enzymes? J. Cell Biol. 104, 1735–1742 (1987).
Dahms, N.M. & Hancock, M.K. P-type lectins. Biochim. Biophys. Acta 1572, 317–340 (2002).
Breuer, P. et al. Serine phosphorylation site of the 46-kDa mannose 6-phosphate receptor is required for transport to the plasma membrane in Madin-Darby canine kidney and mouse fibroblast cells. Mol. Biol. Cell 8, 567–576 (1997).
Beck, M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum. Genet. 121, 1–22 (2007).
Zhu, Y. et al. Carbohydrate-remodelled acid α-glucosidase with higher affinity for the cation-independent mannose 6-phosphate receptor demonstrates improved delivery to muscles of Pompe mice. Biochem. J. 389, 619–628 (2005).
Odani, T., Shimma, Y., Tanaka, A. & Jigami, Y. Cloning and analysis of the MNN4 gene required for phosphorylation of N-linked oligosaccharides in Saccharomyces cerevisiae. Glycobiology 6, 805–810 (1996).
Jigami, Y. & Odani, T. Mannosylphosphate transfer to yeast mannan. Biochim. Biophys. Acta 1426, 335–345 (1999).
Ballou, D.L. Genetic control of yeast mannan structure: mapping genes mnn2 and mnn4 in Saccharomyces cerevisiae. J. Bacteriol. 123, 616–619 (1975).
Akeboshi, H. et al. Production of human β-hexosaminidase A with highly phosphorylated N-glycans by the overexpression of the Ogataea minuta MNN4 gene. Glycobiology 19, 1002–1009 (2009).
York, S.J., Arneson, L.S., Gregory, W.T., Dahms, N.M. & Kornfeld, S. The Rate of Internalization of the mannose 6-phosphate/insulin-like growth factor II receptor is enhanced by multivalent ligand binding. J. Biol. Chem. 274, 1164–1171 (1999).
Struhl, K., Stinchcomb, D.T., Scherer, S. & Davis, R.W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc. Natl. Acad. Sci. USA 76, 1035–1039 (1979).
Scott, J.H. & Schekman, R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J. Bacteriol. 142, 414–423 (1980).
Schwientek, P., Szczepanowski, R., Rückert, C., Stoye, J. & Pühler, A. Sequencing of high G +°C microbial genomes using the ultrafast pyrosequencing technology. J. Biotechnol. 155, 68–77 (2011).
Laroy, W., Contreras, R. & Callewaert, N. Glycome mapping on DNA sequencing equipment. Nat. Protoc. 1, 397–405 (2006).
Takashiba, M., Chiba, Y. & Jigami, Y. Identification of phosphorylation sites in N-linked glycans by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 78, 5208–5213 (2006).
Zhu, Y. et al. Mechanistic insights into a Ca2+-dependent family of alpha-mannosidases in a human gut symbiont. Nat. Chem. Biol. 6, 125–132 (2010).
Lee, K. et al. A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology 13, 305–313 (2003).
Bijvoet, A.G. et al. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum. Mol. Genet. 7, 53–62 (1998).
Zhu, Y. et al. Glycoengineered acid alpha-glucosidase with improved efficacy at correcting the metabolic aberrations and motor function deficits in a mouse model of Pompe disease. Mol. Ther. 17, 954–963 (2009).
Metcalfe, G. & Brown, M.E. Nitrogen fixation by new species of Nocardia. J. Gen. Microbiol. 17, 567–572 (1957).
Schumann, P., Weiss, N. & Stackebrandt, E. Reclassification of Cellulomonas cellulans (Stackebrandt and Keddie 1986) as Cellulosimicrobium cellulans gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 51, 1007–1010 (2001).
Fickers, P. et al. Identification and characterisation of LIP7 and LIP8 genes encoding two extracellular triacylglycerol lipases in the yeast Yarrowia lipolytica. Fungal Genet. Biol. 42, 264–274 (2005).
De Pourcq, K. et al. Engineering the yeast Yarrowia lipolytica for the production of therapeutic proteins homogeneously glycosylated with Man8GlcNAc2 and Man5GlcNAc2. Microb. Cell Fact. 11, 53 (2012).
Vervecken, W. et al. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl. Environ. Microbiol. 70, 2639–2646 (2004).
Barth, G. & Gaillardin, C. Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol. Rev. 19, 219–237 (1997).
Fickers, P., Le Dall, M.T., Gaillardin, C., Thonart, P. & Nicaud, J.M. New disruption cassettes for rapid gene disruption and marker rescue in the yeast Yarrowia lipolytica. J. Microbiol. Methods 55, 727–737 (2003).
Miura, M., Hirose, M., Miwa, T., Kuwae, S. & Ohi, H. Cloning and characterization in Pichia pastoris of PNO1 gene required for phosphomannosylation of N-linked oligosaccharides. Gene 324, 129–137 (2004).
Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 66, 133–144 (2010).
Terwilliger, T.C. et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr. D Biol. Crystallogr. 65, 582–601 (2009).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 17, 490–519 (1996).
Morris, G.M. et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Trott, O. & Olson, A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Reuser, A.J. et al. Uptake and stability of human and bovine acid alpha-glucosidase in cultured fibroblasts and skeletal muscle cells from glycogenosis type II patients. Exp. Cell Res. 155, 178–189 (1984).
Acknowledgements
This work was supported by an R&D grant from IWT-Flanders (projects 080775 and 110379), the Marie Curie Excellence Grant MEXT-014292 under EU Framework Program 6 and by grant G.0.327.11.N.10 of FWO-Vlaanderen. P.T. holds a doctor-assistant position at Ghent University. C.D.V. holds a fellowship of the Institute for the Advancement of Scientific and Technological Research in Industry (IWT). H.R. is supported by VIB grant PRJ9 and the Odysseus program of the FWO-Vlaanderen. We thank J.-M. Nicaud (CNRS-INRA-INAPG UMR2585, France) for donating Y. lipolytica Po1d lnuga, A. Reuser for providing GAA KO mice and TNO Triskelion for executing the in vivo part of the mouse studies. We acknowledge M. Zawisza, D. Bracke, T. Herman, H. Van Put, S. Poiz and I. Dewerte for their excellent technical assistance during the project and thank the Protein Service Facility (Y. Leoen and J. Haustraete) for their assistance, as well as Y.-C. Lin for handling the genome sequencing data. We dedicate this paper to the memory of the late Prof. Dr. Yoshifumi Jigami, one of the pioneers in glyco-engineering of yeasts.
Author information
Authors and Affiliations
Contributions
P.T.: Y. lipolytica MNN4 strain generation, experiments leading to the discovery of enzyme activities in C. cellulans, production of these in E. coli and characterization and thermostability assays. E.B.: CcGH92_5 purification and structure determination. K.P.: CcGH92_4 and CcGH92_5 characterization and enzyme process development, GAA enzyme characterization. C.D.V.: P. pastoris PNO1 and GLA strain generation and characterization. G.P.: cell uptake assays, mouse study bio-analysis. W.N.: docking studies. J.S.: GAA purification, mouse study statistics. F.F.: fermentation, GAA strain generation. P.H.: bio-informatics analysis of CcGH92_5 sequence homologs. S.T.: fermentation development for GAA and CcGH92_4 and CcGH92_5. S.G.: glycan analysis. A.V.H.: Y. lipolytica MNN4 strain generation, production of C. cellulans enzymes in E. coli and thermostability assays. A.V.: Y. lipolytica MNN4 & GAA strain generation. W.V.: Y. lipolytica och1 and MNN4 strain generation, scientific supervision at Oxyrane. H.R.: supervision and analysis of CcGH92 structure determination. N.C.: project initiation and scientific supervision. N.C., P.T., K.P., G.P., C.D.V., W.V. and H.R. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
N.C., W.V., P.T., K.P., A.V., G.P, S.G., J.S. and H.R. are named inventors on patent applications claiming use of the technology described in this publication. W.V., A.V., G.P., S.G. S.T., F.F., J.S. and K.P. hold stock options in Oxyrane UK, a company which develops biopharmaceuticals based partially on technology resulting from the work reported here.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Results, Supplementary Tables 1–5 and Supplementary Figures 1–12 (PDF 6884 kb)
Rights and permissions
About this article
Cite this article
Tiels, P., Baranova, E., Piens, K. et al. A bacterial glycosidase enables mannose-6-phosphate modification and improved cellular uptake of yeast-produced recombinant human lysosomal enzymes. Nat Biotechnol 30, 1225–1231 (2012). https://doi.org/10.1038/nbt.2427
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2427
This article is cited by
-
Glyco-Decipher enables glycan database-independent peptide matching and in-depth characterization of site-specific N-glycosylation
Nature Communications (2022)
-
Customized yeast cell factories for biopharmaceuticals: from cell engineering to process scale up
Microbial Cell Factories (2021)
-
The glycosylation design space for recombinant lysosomal replacement enzymes produced in CHO cells
Nature Communications (2019)
-
Lysosomal Targeting Enhancement by Conjugation of Glycopeptides Containing Mannose-6-phosphate Glycans Derived from Glyco-engineered Yeast
Scientific Reports (2018)
-
Engineering Yarrowia lipolytica for Use in Biotechnological Applications: A Review of Major Achievements and Recent Innovations
Molecular Biotechnology (2018)