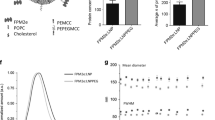
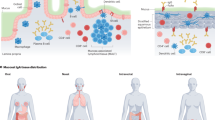
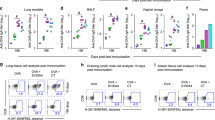
Abstract
Protection against mucosally transmitted infections probably requires immunity at the site of pathogen entry1, yet there are no mucosal adjuvant formulations licensed for human use. Polyethyleneimine (PEI) represents a family of organic polycations used as nucleic acid transfection reagents in vitro and DNA vaccine delivery vehicles in vivo2,3. Here we show that diverse PEI forms have potent mucosal adjuvant activity for viral subunit glycoprotein antigens. A single intranasal administration of influenza hemagglutinin or herpes simplex virus type-2 (HSV-2) glycoprotein D with PEI elicited robust antibody-mediated protection from an otherwise lethal infection, and was superior to existing experimental mucosal adjuvants. PEI formed nanoscale complexes with antigen, which were taken up by antigen-presenting cells in vitro and in vivo, promoted dendritic cell trafficking to draining lymph nodes and induced non-proinflammatory cytokine responses. PEI adjuvanticity required release of host double-stranded DNA that triggered Irf3-dependent signaling. PEI therefore merits further investigation as a mucosal adjuvant for human use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chen, K. & Cerutti, A. Vaccination strategies to promote mucosal antibody responses. Immunity 33, 479–491 (2010).
Gunther, M. et al. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur. J. Pharm. Biopharm. 77, 438–439 (2011).
Lungwitz, U., Breunig, M., Blunk, T. & Gopferich, A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 60, 247–266 (2005).
Lycke, N. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 12, 592–605 (2012).
Fujihashi, K., Koga, T., van Ginkel, F.W., Hagiwara, Y. & McGhee, J.R. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 20, 2431–2438 (2002).
Lewis, D.J. et al. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE 4, e6999 (2009).
Sogaard, O.S. et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin. Infect. Dis. 51, 42–50 (2010).
Heikenwalder, M. et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 10, 187–192 (2004).
Kasturi, S.P. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011).
De Gregorio, E., D'Oro, U. & Wack, A. Immunology of TLR-independent vaccine adjuvants. Curr. Opin. Immunol. 21, 339–345 (2009).
Lambrecht, B.N., Kool, M., Willart, M.A. & Hammad, H. Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 21, 23–29 (2009).
Gavin, A.L. et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 314, 1936–1938 (2006).
Hu, K. et al. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine 29, 1455–1462 (2011).
Ma, Y.F. & Yang, Y.W. Delivery of DNA-based cancer vaccine with polyethylenimine. Eur. J. Pharm. Sci. 40, 75–83 (2010).
Orr, G. et al. Syndecan-1 mediates the coupling of positively charged submicrometer amorphous silica particles with actin filaments across the alveolar epithelial cell membrane. Toxicol. Appl. Pharmacol. 236, 210–220 (2009).
Wegrowski, Y. et al. Cell surface proteoglycan expression during maturation of human monocytes-derived dendritic cells and macrophages. Clin. Exp. Immunol. 144, 485–493 (2006).
Park, K. Luciferin liposomes for enhanced in vivo bioluminescence. J. Control. Release 141, 109 (2009).
Hunter, A.C. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv. Drug Deliv. Rev. 58, 1523–1531 (2006).
Mutsch, M. et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350, 896–903 (2004).
Yamamoto, S. et al. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 94, 5267–5272 (1997).
Lee, J.B., Jang, J.E., Song, M.K. & Chang, J. Intranasal delivery of cholera toxin induces Th17-dominated T-cell response to bystander antigens. PLoS One 4, e5190 (2009).
Lindqvist, M., Persson, J., Thorn, K. & Harandi, A.M. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J. Immunol. 182, 6435–6443 (2009).
Kong, L. et al. Expression-system-dependent modulation of HIV-1 envelope glycoprotein antigenicity and immunogenicity. J. Mol. Biol. 403, 131–147 (2010).
Rudolph, C. et al. Methodological optimization of polyethylenimine (PEI)-based gene delivery to the lungs of mice via aerosol application. J. Gene Med. 7, 59–66 (2005).
Chen, J. et al. Improved antigen cross-presentation by polyethyleneimine-based nanoparticles. Int. J. Nanomedicine 6, 77–84 (2011).
Calabro, S. et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29, 1812–1823 (2011).
Manicassamy, S. & Pulendran, B. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 21, 185–193 (2009).
Marichal, T. et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 17, 996–1002 (2011).
Eisenbarth, S.C., Colegio, O.R., O'Connor, W., Sutterwala, F.S. & Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Torrieri-Dramard, L. et al. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol. Ther. 19, 602–611 (2011).
Littman, D.R. & Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Wegmann, F. et al. A novel strategy for inducing enhanced mucosal HIV-1 antibody responses in an anti-inflammatory environment. PLoS ONE 6, e15861 (2011).
Skehel, J.J. & Waterfield, M.D. Studies on the primary structure of the influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 72, 93–97 (1975).
Del Campo, J. et al. Intranasal immunization with a proteoliposome-derived cochleate containing recombinant gD protein confers protective immunity against genital herpes in mice. Vaccine 28, 1193–1200 (2010).
Sutterwala, F.S. et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006).
Krashias, G. et al. Potent adaptive immune responses induced against HIV-1 gp140 and influenza virus HA by a polyanionic carbomer. Vaccine 28, 2482–2489 (2010).
Gomez Roman, V.R. et al. Development of standard operating procedures to obtain longitudinal vaginal specimens from nulliparous rabbits as part of HIV vaccine mucosal immunogenicity studies. J. Immunol. Methods 363, 29–41 (2010).
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 (1992).
Acknowledgements
This study was supported by grants from The MRC UK, The EU Network of Excellence 'Europrise', The International AIDS Vaccine Initiative Neutralizing Antibody Consortium (IAVI), The Bill and Melinda Gates Foundation Collaboration for Vaccine Design (CAVD), Dormeur Investment Service Ltd., the FP7-funded High Impact Project Advanced Immunization Technologies (ADITEC) and The EuroNanoMed-European Commission funded iNanoDCs. Q.J.S. is a Jenner Institute Investigator and a James Martin Senior Fellow.
Author information
Authors and Affiliations
Contributions
Q.J.S. conceived the study and N.C.S. provided initial proof of concept. F.W., K.H.G., S.A.B., N.C.S., A.M.H., M.C., W.R.H., W.L.K., S.C., T.L., M.P., E.M.S., G.K., A.W. and A.E.M. designed and performed experiments. Q.J.S., F.W. and K.H.G. wrote the paper. A.M.H., L.-P.H., C.S., J.N.B., P.D.G., R.A.F., A.E.M. and N.C.S. supplied reagents and made editorial suggestions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figures 1–12 (PDF 9100 kb)
Rights and permissions
About this article
Cite this article
Wegmann, F., Gartlan, K., Harandi, A. et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol 30, 883–888 (2012). https://doi.org/10.1038/nbt.2344
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2344
This article is cited by
-
An electrostatically conjugated-functional MNK1 aptamer reverts the intrinsic antitumor effect of polyethyleneimine-coated iron oxide nanoparticles in vivo in a human triple-negative cancer xenograft
Cancer Nanotechnology (2023)
-
Engineering nanomaterial physical characteristics for cancer immunotherapy
Nature Reviews Bioengineering (2023)
-
Polymerized porin as a novel delivery platform for coronavirus vaccine
Journal of Nanobiotechnology (2022)
-
Fabrication of subunit nanovaccines by physical interaction
Science China Technological Sciences (2022)
-
A facile approach to enhance antigen response for personalized cancer vaccination
Nature Materials (2018)