Abstract
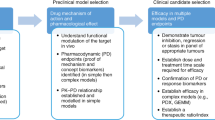
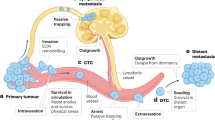
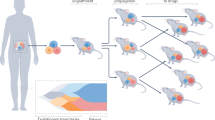
Disappointing results from most late-stage clinical trials of cancer therapeutics indicate a need for improved and more-predictive animal tumor models. This insufficiency of models, combined with the advent of a class of drugs that target the tumor microenvironment rather than the tumor cell, presents new challenges for designing and interpreting preclinical efficacy studies. A comparison of the clinical efficacy of anti-angiogenic drugs with their corresponding preclinical studies over the past two decades offers many lessons that can inform and improve the design of experiments in existing mouse models. In addition, technological and logistical advances in mouse models of human cancer over the past five years have the potential to increase the clinical translatability of animal studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reichert, J.M. & Wenger, J.B. Development trends for new cancer therapeutics and vaccines. Drug Discov. Today 13, 30–37 (2008).
McAllister, S.S. & Weinberg, R.A. Tumor-host interactions: a far-reaching relationship. J. Clin. Oncol. 28, 4022–4028 (2010).
Pietras, K. & Ostman, A. Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell Res. 316, 1324–1331 (2010).
Ferrara, N. & Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005).
Weber, J. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin. Oncol. 37, 430–439 (2010).
Xing, F., Saidou, J. & Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. 15, 166–179 (2010).
Becher, O.J. & Holland, E.C. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 66, 3355–3359 (2006).
Kerbel, R.S. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol. Ther. 2, S134–S139 (2003).
Sausville, E.A. & Burger, A.M. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 66, 3351–3354, discussion 3354 (2006).
Sharpless, N.E. & Depinho, R.A. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat. Rev. Drug Discov. 5, 741–754 (2006).
Singh, M. & Johnson, L. Using genetically engineered mouse models of cancer to aid drug development: an industry perspective. Clin. Cancer Res. 12, 5312–5328 (2006).
Boehm, J.S. & Hahn, W.C. Towards systematic functional characterization of cancer genomes. Nat. Rev. Genet. 12, 487–498 (2011).
Tuveson, D.A. & Jacks, T. Technologically advanced cancer modeling in mice. Curr. Opin. Genet. Dev. 12, 105–110 (2002).
Voskoglou-Nomikos, T., Pater, J.L. & Seymour, L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 9, 4227–4239 (2003).
Wong, K.M., Hudson, T.J. & McPherson, J.D. Unraveling the genetics of cancer: genome sequencing and beyond. Annu. Rev. Genomics Hum. Genet. 12, 407–430 (2011).
Heyer, J., Kwong, L.N., Lowe, S.W. & Chin, L. Non-germline genetically engineered mouse models for translational cancer research. Nat. Rev. Cancer 10, 470–480 (2010).
Bergers, G., Javaherian, K., Lo, K.M., Folkman, J. & Hanahan, D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 284, 808–812 (1999).
Francia, G., Cruz-Munoz, W., Man, S., Xu, P. & Kerbel, R.S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer 11, 135–141 (2011).
Singh, M. et al. Anti-VEGF antibody therapy does not promote metastasis in genetically engineered mouse tumor models. J. Pathol. advance online publication, doi:10.1002/path.4053 (18 May 2012).
Goss, P.E. & Chambers, A.F. Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer 10, 871–877 (2010).
Peterson, J.K. & Houghton, P.J. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur. J. Cancer 40, 837–844 (2004).
Haber, D.A. et al. Molecular targeted therapy of lung cancer: EGFR mutations and response to EGFR inhibitors. Cold Spring Harb. Symp. Quant. Biol. 70, 419–426 (2005).
Wong, H. et al. Pharmacokinetic-pharmacodynamic analysis of vismodegib in preclinical models of mutational and ligand-dependent hedgehog pathway activation. Clin. Cancer Res. 17, 4682–4692 (2011).
Gibbs, J.P. Prediction of exposure-response relationships to support first-in-human study design. AAPS J. 12, 750–758 (2010).
Maziasz, T., Kadambi, V.J., Silverman, L., Fedyk, E. & Alden, C.L. Predictive toxicology approaches for small molecule oncology drugs. Toxicol. Pathol. 38, 148–164 (2010).
Liang, W.C. et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J. Biol. Chem. 281, 951–961 (2006).
Gerber, H.P. et al. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc. Natl. Acad. Sci. USA 104, 3478–3483 (2007).
Ekins, S. & Williams, A.J. Precompetitive preclinical ADME/Tox data: set it free on the web to facilitate computational model building and assist drug development. Lab Chip 10, 13–22 (2010).
Olive, K.P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Van Dyke, T. Finding the tumor copycat: approximating a human cancer. Nat. Med. 16, 976–977 (2010).
Alley, M.C., Hollingshead, M.G., Dykes, D.J. & Waud, W.R. in Anticancer. Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval, 2nd edn. (eds. Teicher, B.A. & Andrews, P.A.) 125–152 (Humana, 2004).
Workman, P. et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 102, 1555–1577 (2010).
de Vries, N.A. et al. Rapid and robust transgenic high-grade glioma mouse models for therapy intervention studies. Clin. Cancer Res. 16, 3431–3441 (2010).
Singh, M. et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat. Biotechnol. 28, 585–593 (2010).
LoRusso, P.M., Anderson, A.B., Boerner, S.A. & Averbuch, S.D. Making the investigational oncology pipeline more efficient and effective: are we headed in the right direction? Clin. Cancer Res. 16, 5956–5962 (2010).
Teicher, B.A. Antiangiogenic agents and targets: a perspective. Biochem. Pharmacol. 81, 6–12 (2011).
Ebos, J.M. & Kerbel, R.S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol. 8, 210–221 (2011).
Folkman, J., Watson, K., Ingber, D. & Hanahan, D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339, 58–61 (1989).
Zucker, S., Cao, J. & Chen, W.T. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 19, 6642–6650 (2000).
Coussens, L.M., Fingleton, B. & Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295, 2387–2392 (2002).
Overall, C.M. & Kleifeld, O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 6, 227–239 (2006).
Roy, R., Yang, J. & Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 27, 5287–5297 (2009).
Folkman, J. Antiangiogenesis in cancer therapy–endostatin and its mechanisms of action. Exp. Cell Res. 312, 594–607 (2006).
Boehm, T., Folkman, J., Browder, T. & O'Reilly, M.S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 390, 404–407 (1997).
Karamouzis, M.V. & Moschos, S.J. The use of endostatin in the treatment of solid tumors. Expert Opin. Biol. Ther. 9, 641–648 (2009).
Kulke, M.H. et al. Phase II study of recombinant human endostatin in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 24, 3555–3561 (2006).
Bhargava, P. et al. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin. Cancer Res. 5, 1989–1995 (1999).
Kim, K.J. et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362, 841–844 (1993).
Mesiano, S., Ferrara, N. & Jaffe, R.B. Role of vascular endothelial growth factor in ovarian cancer: Inhibition of ascites formation by immunoneutralization. Am. J. Pathol. 15, 1249–1256 (1998).
Allegra, C.J. et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J. Clin. Oncol. 29, 11–16 (2011).
Burger, R.A. et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 365, 2473–2483 (2011).
Perren, T.J. et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 365, 2484–2496 (2011).
Pietras, K. & Hanahan, D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J. Clin. Oncol. 23, 939–952 (2005).
Raymond, E. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 501–513 (2011).
Ebos, J.M. et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Sennino, B. et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2, 270–287 (2012).
Crawford, Y. & Ferrara, N. Tumor- and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol. Sci. 30, 624–630 (2009).
Bergers, G., Song, S., Meyer-Morse, N., Bergsland, E. & Hanahan, D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 111, 1287–1295 (2003).
Xian, X. et al. Pericytes limit tumor cell metastasis. J. Clin. Invest. 116, 642–651 (2006).
Cooke, V.G. et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by Met signaling pathway. Cancer Cell 21, 66–81 (2012).
Miles, D. et al. Disease course patterns after discontinuation of bevacizumab: pooled analysis of randomized phase III trials. J. Clin. Oncol. 29, 83–88 (2011).
Rubenstein, J.L. et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia 2, 306–314 (2000).
Hanahan, D. & Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
de la Cruz-Merino, L., Grande-Pulido, E., Albero-Tamarit, A. & Codes-Manuel de Villena, M.E. Cancer and immune response: old and new evidence for future challenges. Oncologist 13, 1246–1254 (2008).
Tivol, E.A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Melero, I., Hervas-Stubbs, S., Glennie, M., Pardoll, D.M. & Chen, L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer 7, 95–106 (2007).
Kwon, E.D. et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA 94, 8099–8103 (1997).
Leach, D.R., Krummel, M.F. & Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
van Elsas, A., Hurwitz, A.A. & Allison, J.P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Mokyr, M.B., Kalinichenko, T., Gorelik, L. & Bluestone, J.A. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 58, 5301–5304 (1998).
Phan, G.Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 100, 8372–8377 (2003).
Hodi, F.S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Wolchok, J.D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Sharma, P., Wagner, K., Wolchok, J.D. & Allison, J.P. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat. Rev. Cancer 11, 805–812 (2011).
Callahan, M.K., Wolchok, J.D. & Allison, J.P. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin. Oncol. 37, 473–484 (2010).
Hoos, A. et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin. Oncol. 37, 533–546 (2010).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Topalian, S.L. et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New Engl. J. Med. published online, doi:10.1056/NEJMoa1200690 (2 June 2012).
Brahmer, J.R. et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. New Engl. J. Med. published online, doi: 10.1056/NEJMoa1200694 (2 June 2012).
Tlsty, T.D. & Coussens, L.M. Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. 1, 119–150 (2006).
Crawford, Y. et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15, 21–34 (2009).
Gilbert, L.A. & Hemann, M.T. DNA damage-mediated induction of a chemoresistant niche. Cell 143, 355–366 (2010).
Andreu, P. et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17, 121–134 (2010).
Coussens, L.M. et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 13, 1382–1397 (1999).
Olumi, A.F. et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 59, 5002–5011 (1999).
Shojaei, F. et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 25, 911–920 (2007).
Erez, N., Truitt, M., Olson, P., Arron, S.T. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting unflammation in an NF-κB-dependent manner. Cancer Cell 17, 135–147 (2010).
Gilbert, L.A. & Hemann, M.T. Chemotherapeutic resistance: surviving stressful situations. Cancer Res. 71, 5062–5066 (2011).
Teicher, B.A. In vivo/ex vivo and in situ assays used in cancer research: a brief review. Toxicol. Pathol. 37, 114–122 (2009).
Jia, J. et al. Mechanisms of drug combinations: interaction and network perspectives. Nat. Rev. Drug Discov. 8, 111–128 (2009).
Rottenberg, S. & Jonkers, J. Modeling therapy resistance in genetically engineered mouse cancer models. Drug Resist. Updat. 11, 51–60 (2008).
Zuber, J. et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 23, 877–889 (2009).
Pajic, M. et al. Moderate increase in Mdr1a/1b expression causes in vivo resistance to doxorubicin in a mouse model for hereditary breast cancer. Cancer Res. 69, 6396–6404 (2009).
Politi, K., Fan, P.D., Shen, R., Zakowski, M. & Varmus, H. Erlotinib resistance in mouse models of epidermal growth factor receptor-induced lung adenocarcinoma. Dis. Model Mech. 3, 111–119 (2010).
Sequist, L.V. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 3, 75ra26 (2011).
Sharma, S.V., Haber, D.A. & Settleman, J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat. Rev. Cancer 10, 241–253 (2010).
Zhou, Y. et al. Chimeric mouse tumor models reveal differences in pathway activation between ERBB family- and KRAS-dependent lung adenocarcinomas. Nat. Biotechnol. 28, 71–78 (2010).
Shojaei, F. et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 (2007).
Allen, E., Walters, I. & Hanahan, D. Brivanib, an FGF/VEGF inhibitor, is differentially active 1st vs. 2nd line against mouse PNET tumors developing evasive/adaptive resistance to VEGF inhibition. Clin. Cancer Res. 17, 5299–5310 (2011).
Casanovas, O., Hicklin, D.J., Bergers, G. & Hanahan, D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8, 299–309 (2005).
Compagni, A., Wilgenbus, P., Impagnatiello, M.A., Cotten, M. & Christofori, G. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res. 60, 7163–7169 (2000).
Begley, C.G. & Ellis, L.M. Drug development: raise standards for preclinical cancer research. Nature 483, 531–533 (2012).
Maxmen, A. Translational research: the American way. Nature 478, S16–S18 (2011).
Schilsky, R.L. Accrual to cancer clinical trials in the era of molecular medicine. Sci. Transl. Med. 3, 75cm9 (2011).
Yap, T.A., Sandhu, S.K., Workman, P. & de Bono, J.S. Envisioning the future of early anticancer drug development. Nat. Rev. Cancer 10, 514–523 (2010).
Taguchi, A. et al. Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Cancer Cell 20, 289–299 (2011).
Chen, Z. et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 483, 613–617 (2012).
Hanash, S.M., Baik, C.S. & Kallioniemi, O. Emerging molecular biomarkers–blood-based strategies to detect and monitor cancer. Nat. Rev. Clin. Oncol. 8, 142–150 (2011).
Mina, L.A. & Sledge, G.W. Jr. Rethinking the metastatic cascade as a therapeutic target. Nat. Rev. Clin. Oncol. 8, 325–332 (2011).
Premsrirut, P.K. et al. A rapid and scalable system for studying gene function in mice using conditional RNA interference. Cell 145, 145–158 (2011).
Schreiber, S.L. et al. Towards patient-based cancer therapeutics. Nat. Biotechnol. 28, 904–906 (2010).
Doyle, J.P. et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135, 749–762 (2008).
Heiman, M. et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell 135, 738–748 (2008).
Sanz, E. et al. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA 106, 13939–13944 (2009).
Lesterhuis, W.J., Haanen, J.B. & Punt, C.J. Cancer immunotherapy–revisited. Nat. Rev. Drug Discov. 10, 591–600 (2011).
Scher, H.I., Nasso, S.F., Rubin, E.H. & Simon, R. Adaptive clinical trial designs for simultaneous testing of matched diagnostics and therapeutics. Clin. Cancer Res. 17, 6634–6640 (2011).
Sharma, M.R., Stadler, W.M. & Ratain, M.J. Randomized phase II trials: a long-term investment with promising returns. J. Natl. Cancer Inst. 103, 1093–1100 (2011).
Gerber, H.P. & Ferrara, N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 65, 671–680 (2005).
Bruce, D. & Tan, P.H. Blocking the interaction of vascular endothelial growth factor receptors with their ligands and their effector signaling as a novel therapeutic target for cancer: time for a new look? Expert Opin. Investig. Drugs 20, 1413–1434 (2011).
Cao, Y. Antiangiogenic cancer therapy: why do mouse and human patients respond in a different way to the same drug? Int. J. Dev. Biol. 55, 557–562 (2011).
Gandhi, L. et al. Sunitinib prolongs survival in genetically engineered mouse models of multistep lung carcinogenesis. Cancer Prev. Res. (Phila.) 2, 330–337 (2009).
Scagliotti, G. et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J. Clin. Oncol. 28, 1835–1842 (2010).
Kindler, H.L. et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 28, 3617–3622 (2010).
Kindler, H.L. et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 23, 8033–8040 (2005).
Olson, P., Chu, G.C., Perry, S.R., Nolan-Stevaux, O. & Hanahan, D. Imaging guided trials of the angiogenesis inhibitor sunitinib in mouse models predict efficacy in pancreatic neuroendocrine but not ductal carcinoma. Proc. Natl. Acad. Sci. USA 108, E1275–E1284 (2011).
Kindler, H.L. et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 12, 256–262 (2011).
Acknowledgements
We thank A. Bruce for graphics, and A. Polson and C. Bais for discussions, input and insights.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
M.S. is an employee of Novartis. N.F. is an employee of Genentech/Roche.
Rights and permissions
About this article
Cite this article
Singh, M., Ferrara, N. Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nat Biotechnol 30, 648–657 (2012). https://doi.org/10.1038/nbt.2286
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2286
This article is cited by
-
Retinoic acid inhibits the angiogenesis of human embryonic stem cell-derived endothelial cells by activating FBP1-mediated gluconeogenesis
Stem Cell Research & Therapy (2022)
-
Tumor microenvironment heterogeneity an important mediator of prostate cancer progression and therapeutic resistance
npj Precision Oncology (2022)
-
Hepatocellular carcinoma
Nature Reviews Disease Primers (2021)
-
Drug resistance profiling of a new triple negative breast cancer patient-derived xenograft model
BMC Cancer (2019)
-
Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment
Scientific Reports (2018)