Abstract
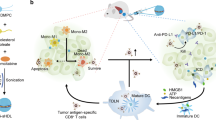
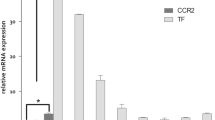
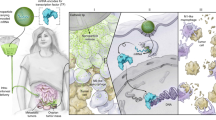
Excessive and prolonged activity of inflammatory monocytes is a hallmark of many diseases with an inflammatory component. In such conditions, precise targeting of these cells could be therapeutically beneficial while sparing many essential functions of the innate immune system, thus limiting unwanted effects. Inflammatory monocytes—but not the noninflammatory subset—depend on the chemokine receptor CCR2 for localization to injured tissue. Here we present an optimized lipid nanoparticle and a CCR2-silencing short interfering RNA that, when administered systemically in mice, show rapid blood clearance, accumulate in spleen and bone marrow, and localize to monocytes. Efficient degradation of CCR2 mRNA in monocytes prevents their accumulation in sites of inflammation. Specifically, the treatment attenuates their number in atherosclerotic plaques, reduces infarct size after coronary artery occlusion, prolongs normoglycemia in diabetic mice after pancreatic islet transplantation, and results in reduced tumor volumes and lower numbers of tumor-associated macrophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Charo, I.F. & Ransohoff, R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Serbina, N.V. & Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Gu, L. et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 2, 275–281 (1998).
Boring, L., Gosling, J., Cleary, M. & Charo, I.F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998).
Dewald, O. et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 96, 881–889 (2005).
Kaikita, K. et al. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am. J. Pathol. 165, 439–447 (2004).
Abdi, R. et al. Differential role of CCR2 in islet and heart allograft rejection: tissue specificity of chemokine/chemokine receptor function in vivo. J. Immunol. 172, 767–775 (2004).
Lu, X. & Kang, Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J. Biol. Chem. 284, 29087–29096 (2009).
Aouadi, M. et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 458, 1180–1184 (2009).
Peer, D., Park, E.J., Morishita, Y., Carman, C.V. & Shimaoka, M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319, 627–630 (2008).
Whitehead, K.A., Langer, R. & Anderson, D.G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 8, 129–138 (2009).
Judge, A.D. et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J. Clin. Invest. 119, 661–673 (2009).
Love, K.T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl. Acad. Sci. USA 107, 1864–1869 (2010).
Swirski, F.K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Nahrendorf, M., Pittet, M.J. & Swirski, F.K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010).
Swirski, F.K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Robertson, R.P. Islet transplantation as a treatment for diabetes - a work in progress. N. Engl. J. Med. 350, 694–705 (2004).
Swirski, F.K. et al. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J. Clin. Invest. 120, 2627–2634 (2010).
Grivennikov, S.I., Greten, F.R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Qian, B.Z. & Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
De Palma, M. & Lewis, C.E. Cancer: macrophages limit chemotherapy. Nature 472, 303–304 (2011).
Qian, B.Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Movahedi, K. et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 70, 5728–5739 (2010).
Steidl, C. et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N. Engl. J. Med. 362, 875–885 (2010).
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 (2007).
Hart, K.M., Bak, S.P., Alonso, A. & Berwin, B. Phenotypic and functional delineation of murine CX(3)CR1 monocyte-derived cells in ovarian cancer. Neoplasia 11, 564–573 (2009).
Pahler, J.C. et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia 10, 329–340 (2008).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Weissleder, R. & Pittet, M.J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Vaishnaw, A.K. et al. A status report on RNAi therapeutics. Silence 1, 14 (2010).
Roberts, R., DeMello, V. & Sobel, B.E. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation 53, I204–I206 (1976).
Buxton, D.B. Nanotechnology research support at the national heart, lung, and blood institute. Circ. Res. 109, 250–254 (2011).
Frank-Kamenetsky, M. et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA 105, 11915–11920 (2008).
Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 26, 561–569 (2008).
Heyes, J., Palmer, L., Bremner, K. & MacLachlan, I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Control. Release 107, 276–287 (2005).
Nahrendorf, M. et al. Hybrid PET-optical imaging using targeted probes. Proc. Natl. Acad. Sci. USA 107, 7910–7915 (2010).
Leuschner, F. et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ. Res. 107, 1364–1373 (2010).
Moore, D.J. et al. Resistance to anti-CD45RB-induced tolerance in NOD mice: mechanisms involved. Transpl. Int. 17, 261–269 (2004).
Souto, F.O. et al. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am. J. Respir. Crit. Care Med. 183, 234–242 (2011).
Furuichi, K. et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J. Am. Soc. Nephrol. 14, 2503–2515 (2003).
Acknowledgements
The authors thank M. Waring, A. Chicoine and the Ragon Institute (MGH) for cell sorting, the CSB Mouse Imaging Program (P. Waterman, B. Sena) and B. Bettencourt for designing initial sets of siRNA. We acknowledge the small, medium and large scale RNA synthesis groups at Alnylam as well as analytical, duplex annealing and QC groups for synthesizing and characterizing RNAs. This work was funded in part by grants from the US National Institutes of Health R01-HL096576, R01-HL095629 (M.N.); R01-EB006432, T32-CA79443, U24-CA92782, P50-CA86355, HHSN268201000044C (R.W.); R01-CA132091, R01-CA115527, R37-EB000244 (R.L.); Deutsche Herzstiftung (F.L.); and the SNUBH Research Fund 02-2007-013 (W.W.L.).
Author information
Authors and Affiliations
Contributions
F.L. and P.D. performed experiments, collected and analyzed the data and contributed to writing the manuscript, R.G. did surgeries and performed experiments, T.I.N. designed experiments and siRNA screens, analyzed data and contributed to writing the manuscript; K.M.L. did islet transplantations and analyzed data, J.S.D., G.C., J.I.K., J.F.M., B.M., P.P., W.W.L., Y.I., V.C.-R., A.N., W.C., J.W. performed experiments, imaging, collected, analyzed and discussed data, S.M., H.E.-B., K.L. formulated siRNA nanoparticles, P.L., M.J.P. and F.K.S. conceived experiments and discussed strategy and results; V.K., R.L., R.W., D.G.A. and M.N. designed experiments, developed siRNA delivery technology and in vivo imaging strategies and systems, and reviewed, analyzed and discussed data. M.N. and R.W. wrote the manuscript which was edited and approved by all co-authors. M.N. developed and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
T.I.N., S.M., H.E.B., W.C., J.W. and V.K. are Alnylam Pharmaceuticals employees; K.L., R.L., and D.G.A. receive funding from Alnylam Pharmaceuticals. R.L. and D.G.A. are consultants with Alnylam.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 (PDF 3232 kb)
Rights and permissions
About this article
Cite this article
Leuschner, F., Dutta, P., Gorbatov, R. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 29, 1005–1010 (2011). https://doi.org/10.1038/nbt.1989
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1989
This article is cited by
-
Strategies for non-viral vectors targeting organs beyond the liver
Nature Nanotechnology (2024)
-
Nano–Bio Interactions: Exploring the Biological Behavior and the Fate of Lipid-Based Gene Delivery Systems
BioDrugs (2024)
-
Macrophage profiling in atherosclerosis: understanding the unstable plaque
Basic Research in Cardiology (2024)
-
Macrophage-based therapeutic approaches for cardiovascular diseases
Basic Research in Cardiology (2024)
-
Targeting RNA N6-methyladenosine to synergize with immune checkpoint therapy
Molecular Cancer (2023)