Abstract
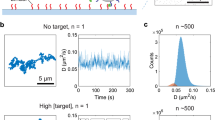
Molecular biomarkers can be used as objective indicators of pathologic processes. Although their levels often change over time, their measurement is often constrained to a single time point. Cumulative biomarker exposure would provide a fundamentally different kind of measurement to what is available in the clinic. Magnetic resonance relaxometry can be used to noninvasively monitor changes in the relaxation properties of antibody-coated magnetic particles when they aggregate upon exposure to a biomarker of interest. We used implantable devices containing such sensors to continuously profile changes in three clinically relevant cardiac biomarkers at physiological levels for up to 72 h. Sensor response differed between experimental and control groups in a mouse model of myocardial infarction and correlated with infarct size. Our prototype for a biomarker monitoring device also detected doxorubicin-induced cardiotoxicity and can be adapted to detect other molecular biomarkers with a sensitivity as low as the pg/ml range.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gutterman, D.D. Silent myocardial ischemia. Circ. J. 73, 785–797 (2009).
Sheifer, S.E., Manolio, T.A. & Gersh, B.J. Unrecognized myocardial infarction. Ann. Intern. Med. 135, 801–811 (2001).
Ammar, K.A., Kors, J.A., Yawn, B.P. & Rodeheffer, R.J. Defining unrecognized myocardial infarction: a call for standardized electrocardiographic diagnostic criteria. Am. Heart J. 148, 277–284 (2004).
Shapiro, M.G., Atanasijevic, T., Faas, H., Westmeyer, G.G. & Jasanoff, A. Dynamic imaging with MRI contrast agents: quantitative considerations. Magn. Reson. Imaging 24, 449–462 (2006).
Daniel, K.D. et al. Multi-reservoir device for detecting a soluble cancer biomarker. Lab Chip 7, 1288–1293 (2007).
Kim, G.Y., Josephson, L., Langer, R. & Cima, M.J. Magnetic relaxation switch detection of human chorionic gonadotrophin. Bioconjug. Chem. 18, 2024–2028 (2007).
Taktak, S., Sosnovik, D., Cima, M.J., Weissleder, R. & Josephson, L. Multiparameter magnetic relaxation switch assays. Anal. Chem. 79, 8863–8869 (2007).
Tsourkas, A., Hofstetter, O., Hofstetter, H., Weissleder, R. & Josephson, L. Magnetic relaxation switch immunosensors detect enantiomeric impurities. Angew. Chem. Int. Edn Engl. 43, 2395–2399 (2004).
Perez, J.M., Josephson, L., O'Loughlin, T., Hogemann, D. & Weissleder, R. Magnetic relaxation switches capable of sensing molecular interactions. Nat. Biotechnol. 20, 816–820 (2002).
Perez, J.M., Josephson, L. & Weissleder, R. Use of magnetic nanoparticles as nanosensors to probe for molecular interactions. ChemBioChem 5, 261–264 (2004).
Perez, J.M., O'Loughin, T., Simeone, F.J., Weissleder, R. & Josephson, L. DNA-based magnetic nanoparticle assembly acts as a magnetic relaxation nanoswitch allowing screening of DNA-cleaving agents. J. Am. Chem. Soc. 124, 2856–2857 (2002).
Sun, E.Y., Weissleder, R. & Josephson, L. Continuous analyte sensing with magnetic nanoswitches. Small 2, 1144–1147 (2006).
Wunderbaldinger, P., Josephson, L. & Weissleder, R. Crosslinked iron oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad. Radiol. 9 Suppl 2, S304–S306 (2002).
Zhao, M., Josephson, L., Tang, Y. & Weissleder, R. Magnetic sensors for protease assays. Angew. Chem. Int. Edn Engl. 42, 1375–1378 (2003).
Daniel, K.D. et al. Implantable diagnostic device for cancer monitoring. Biosens. Bioelectron. 24, 3252–3257 (2009).
Jaffe, A.S., Babuin, L. & Apple, F.S. Biomarkers in acute cardiac disease: the present and the future. J. Am. Coll. Cardiol. 48, 1–11 (2006).
Tarnavski, O. et al. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol. Genomics 16, 349–360 (2004).
Thygesen, K., Alpert, J.S. & White, H.D. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 50, 2173–2195 (2007).
Scherrer-Crosbie, M., Rodrigues, A.C.T., Hataishi, R. & Picard, M.H. Infarct size assessment in mice. Echocardiography 24, 90–96 (2007).
Bello, D. et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J. Am. Coll. Cardiol. 45, 1104–1108 (2005).
Roes, S.D. et al. Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am. J. Cardiol. 100, 930–936 (2007).
Takemura, G. & Fujiwara, H. Doxorubicin-induced cardiomyopathy: from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 49, 330–352 (2007).
Robert, J. Long-term and short-term models for studying anthracycline cardiotoxicity and protectors. Cardiovasc. Toxicol. 7, 135–139 (2007).
Wallace, K.B. et al. Serum troponins as biomarkers of drug-induced cardiac toxicity. Toxicol. Pathol. 32, 106–121 (2004).
Josephson, L., Tung, C.H., Moore, A. & Weissleder, R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug. Chem. 10, 186–191 (1999).
Acknowledgements
This work was supported by National Cancer Institute Centers of Cancer Nanotechnology Excellence no. 5 U54 CA119349-12 and CA151844 grants and National Science Foundation Division of Materials Research Award no. 0746264. Y.L. was supported by a National Defense Science and Engineering Graduate fellowship. T.P. was supported by an American Heart Association fellowship.
Author information
Authors and Affiliations
Contributions
Y.L. initiated the project, designed and performed experiments, analyzed data and wrote the manuscript. T.P. conceived experiments, designed and performed animal experiments, analyzed data and wrote the manuscript. C.C.V. contributed ideas, performed experiments, analyzed data and wrote the manuscript. P.L.H. contributed to the design experiments related to clinical relevance, doxorubicin toxicity and myocardial infarction model. M.J.C. was the principal investigator; he initiated the project, conceived experiments and obtained funding.
Corresponding author
Ethics declarations
Competing interests
M.J.C. is a director at T2 Biosystems, a company developing in vitro diagnostic assays.
Rights and permissions
About this article
Cite this article
Ling, Y., Pong, T., Vassiliou, C. et al. Implantable magnetic relaxation sensors measure cumulative exposure to cardiac biomarkers. Nat Biotechnol 29, 273–277 (2011). https://doi.org/10.1038/nbt.1780
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1780
This article is cited by
-
Magnetic Nanoparticles for Multi-Imaging and Drug Delivery
Molecules and Cells (2013)
-
Our choice from the recent literature
Nature Materials (2011)
-
Biomarkers in aggregate
Nature Biotechnology (2011)