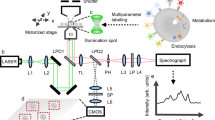
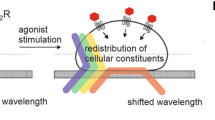
Abstract
Label-free biosensor technology based on dynamic mass redistribution (DMR) of cellular constituents promises to translate GPCR signaling into complex optical 'fingerprints' in real time in living cells. Here we present a strategy to map cellular mechanisms that define label-free responses, and we compare DMR technology with traditional second-messenger assays that are currently the state of the art in GPCR drug discovery. The holistic nature of DMR measurements enabled us to (i) probe GPCR functionality along all four G-protein signaling pathways, something presently beyond reach of most other assay platforms; (ii) dissect complex GPCR signaling patterns even in primary human cells with unprecedented accuracy; (iii) define heterotrimeric G proteins as triggers for the complex optical fingerprints; and (iv) disclose previously undetected features of GPCR behavior. Our results suggest that DMR technology will have a substantial impact on systems biology and systems pharmacology as well as for the discovery of drugs with novel mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Overington, J.P., Al-Lazikani, B. & Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006).
Baker, J.G. & Hill, S.J. Multiple GPCR conformations and signalling pathways: implications for antagonist affinity estimates. Trends Pharmacol. Sci. 28, 374–381 (2007).
Bosier, B. & Hermans, E. Versatility of GPCR recognition by drugs: from biological implications to therapeutic relevance. Trends Pharmacol. Sci. 28, 438–446 (2007).
Galandrin, S., Oligny-Longpre, G. & Bouvier, M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol. Sci. 28, 423–430 (2007).
Kenakin, T.P. Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat. Rev. Drug Discov. 8, 617–626 (2009).
Urban, J.D. et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 (2007).
Kenakin, T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol. Sci. 16, 232–238 (1995).
Ryberg, E. et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152, 1092–1101 (2007).
Eglen, R.M. Functional G protein-coupled receptor assays for primary and secondary screening. Comb. Chem. High Throughput Screen. 8, 311–318 (2005).
Williams, C. cAMP detection methods in HTS: selecting the best from the rest. Nat. Rev. Drug Discov. 3, 125–135 (2004).
Willoughby, D. & Cooper, D.M. Live-cell imaging of cAMP dynamics. Nat. Methods 5, 29–36 (2008).
Barnea, G. et al. The genetic design of signaling cascades to record receptor activation. Proc. Natl. Acad. Sci. USA 105, 64–69 (2008).
Hamdan, F.F., Audet, M., Garneau, P., Pelletier, J. & Bouvier, M. High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based β-arrestin2 recruitment assay. J. Biomol. Screen. 10, 463–475 (2005).
Lefkowitz, R.J. & Whalen, E.J. β-arrestins: traffic cops of cell signaling. Curr. Opin. Cell Biol. 16, 162–168 (2004).
Olson, K.R. & Eglen, R.M. β-galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev. Technol. 5, 137–144 (2007).
Verkaar, F. et al. G protein-independent cell-based assays for drug discovery on seven-transmembrane receptors. Biotechnol. Annu. Rev. 14, 253–274 (2008).
Azzi, M. et al. β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 100, 11406–11411 (2003).
Galandrin, S. & Bouvier, M. Distinct signaling profiles of β1 and β2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol. Pharmacol. 70, 1575–1584 (2006).
Hansen, J.L., Theilade, J., Haunso, S. & Sheikh, S.P. Oligomerization of wild type and nonfunctional mutant angiotensin II type I receptors inhibits Gαq protein signaling but not ERK activation. J. Biol. Chem. 279, 24108–24115 (2004).
Hoffmann, C., Ziegler, N., Reiner, S., Krasel, C. & Lohse, M.J. Agonist-selective, receptor-specific interaction of human P2Y receptors with β-arrestin-1 and -2. J. Biol. Chem. 283, 30933–30941 (2008).
Violin, J.D. & Lefkowitz, R.J. β-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 28, 416–422 (2007).
Fang, Y., Ferrie, A.M., Fontaine, N.H., Mauro, J. & Balakrishnan, J. Resonant waveguide grating biosensor for living cell sensing. Biophys. J. 91, 1925–1940 (2006).
Fang, Y., Li, G. & Ferrie, A.M. Non-invasive optical biosensor for assaying endogenous G protein-coupled receptors in adherent cells. J. Pharmacol. Toxicol. Methods 55, 314–322 (2007).
Fang, Y. & Ferrie, A.M. Optical biosensor differentiates signaling of endogenous PAR1 and PAR2 in A431 cells. BMC Cell Biol. 8, 24 (2007).
Rocheville, M. & Jerman, J.C. 7TM pharmacology measured by label-free: a holistic approach to cell signalling. Curr. Opin. Pharmacol. 9, 643–649 (2009).
Dodgson, K., Gedge, L., Murray, D.C. & Coldwell, M. A 100K well screen for a muscarinic receptor using the Epic label-free system—a reflection on the benefits of the label-free approach to screening seven-transmembrane receptors. J. Recept. Signal Transduct. Res. 29, 163–172 (2009).
Lee, P.H. et al. Evaluation of dynamic mass redistribution technology for pharmacological studies of recombinant and endogenously expressed G protein-coupled receptors. Assay Drug Dev. Technol. 6, 83–94 (2008).
McGuinness, R.P. et al. Enhanced selectivity screening of GPCR ligands using a label-free cell based assay technology. Comb. Chem. High Throughput Screen. 12, 812–823 (2009).
Peters, M.F., Vaillancourt, F., Heroux, M., Valiquette, M. & Scott, C.W. Comparing label-free biosensors for pharmacological screening with cell-based functional assays. Assay Drug Dev. Technol. 8, 219–227 (2010).
Takasaki, J. et al. A novel Gαq/11-selective inhibitor. J. Biol. Chem. 279, 47438–47445 (2004).
Riobo, N.A. & Manning, D.R. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol. Sci. 26, 146–154 (2005).
Henstridge, C.M. et al. The GPR55 ligand l-α-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 23, 183–193 (2009).
Ross, R.A. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 30, 156–163 (2009).
Henstridge, C.M. et al. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br. J. Pharmacol. 160, 604–614 (2010).
Briscoe, C.P. et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278, 11303–11311 (2003).
Itoh, Y. et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422, 173–176 (2003).
Stoddart, L.A., Brown, A.J. & Milligan, G. Uncovering the pharmacology of the G protein-coupled receptor GPR40: high apparent constitutive activity in guanosine 5′-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist. Mol. Pharmacol. 71, 994–1005 (2007).
Christiansen, E. et al. Discovery of potent and selective agonists for the free fatty acid receptor 1 (FFA(1)/GPR40), a potential target for the treatment of type II diabetes. J. Med. Chem. 51, 7061–7064 (2008).
Zhang, J.Z., Maruyama, K., Iwatsuki, K., Ono, I. & Kaneko, F. Effects of prostaglandin E1 on human keratinocytes and dermal fibroblasts: a possible mechanism for the healing of skin ulcers. Exp. Dermatol. 3, 164–170 (1994).
McGraw, D.W., Almoosa, K.F., Paul, R.J., Kobilka, B.K. & Liggett, S.B. Antithetic regulation by β-adrenergic receptors of Gq receptor sinaling via phospholipase C underlies the airway beta-agonist paradox. J. Clin. Invest. 112, 619–626 (2003).
McGuinness, R. Impedance-based cellular assay technologies: recent advances, future promise. Curr. Opin. Pharmacol. 7, 535–540 (2007).
Gaspard, N. et al. Generation of cortical neurons from mouse embryonic stem cells. Nat. Protoc. 4, 1454–1463 (2009).
James, D. et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFβ inhibition is Id1 dependent. Nat. Biotechnol. 28, 161–166 (2010).
Burford, N.T., Tobin, A.B. & Nahorski, S.R. Differential coupling of m1, m2 and m3 muscarinic receptor subtypes to inositol 1,4,5-trisphosphate and adenosine 3′,5′-cyclic monophosphate accumulation in Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 274, 134–142 (1995).
Schmidt, M., Nehls, C., Rumenapp, U. & Jakobs, K.H. m3 Muscarinic receptor-induced and Gi-mediated heterologous potentiation of phospholipase C stimulation: role of phosphoinositide synthesis. Mol. Pharmacol. 50, 1038–1046 (1996).
Pantaloni, C. et al. Alternative splicing in the N-terminal extracellular domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor modulates receptor selectivity and relative potencies of PACAP-27 and PACAP-38 in phospholipase C activation. J. Biol. Chem. 271, 22146–22151 (1996).
Antony, J. et al. Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity. FASEB J. 23, 442–450 (2009).
Jäger, D. et al. Allosteric small molecules unveil a role of an extracellular E2/transmembrane helix 7 junction for G protein-coupled receptor activation. J. Biol. Chem. 282, 34968–34976 (2007).
Smith, N.J., Stoddart, L.A., Devine, N.M., Jenkins, L. & Milligan, G. The action and mode of Binding of thiazolidinedione ligands at free fatty acid receptor 1. J. Biol. Chem. 284, 17527–17539 (2009).
Schröder, R. et al. The C-terminal tail of CRTH2 is a key molecular determinant that constrains Gαi and downstream signaling cascade activation. J. Biol. Chem. 284, 1324–1336 (2009).
Acknowledgements
We thank U. Rick, M. Vasmer-Ehses and T. Kögler for expert technical assistance and Corning Inc. for providing us with the Epic system. This work was supported by the DFG (Deutsche Forschungsgemeinschaft) grants KO 1582/3-1 to E.K., MO 821/2-1 to K.M. and WE 4428/1-1 to J.W. and by the Dr. Hilmer Foundation (PhD fellowship to S.H.). A.K. is a member of the DFG-funded Research Training School GRK 677. N.J.S is an NHMRC/NHF of Australia Overseas Research Fellow. We thank M. De Amici and U. Holzgrabe (University of Milan and University of Würzburg) for kindly providing Hybrid 1 (ref. 47), T. Ulven (University of Southern Denmark) for TUG424 (ref. 38) and Astellas Pharma Inc. (Osaka, Japan) for providing us with YM-254890 (ref. 30).
Author information
Authors and Affiliations
Contributions
R.S., N.J., J.S., A.K., N.M., S.H., A.M., S.B., M.M.-A. and N.J.S. designed and performed the experiments; E.K. and K.M. designed the research and wrote the manuscript; S.Z., J.W. and G.M. provided important biological samples or research tools; C.D., J.G., N.J.S., G.M. and R.S. provided important ideas and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The Epic biosensor used in the procedure described in this article was provided to E.K. by Corning Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 (PDF 398 kb)
Rights and permissions
About this article
Cite this article
Schröder, R., Janssen, N., Schmidt, J. et al. Deconvolution of complex G protein–coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol 28, 943–949 (2010). https://doi.org/10.1038/nbt.1671
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1671
This article is cited by
-
GPCR kinase knockout cells reveal the impact of individual GRKs on arrestin binding and GPCR regulation
Nature Communications (2022)
-
Investigating the ligand agonism and antagonism at the D2long receptor by dynamic mass redistribution
Scientific Reports (2022)
-
Heterocomplexes between the atypical chemokine MIF and the CXC-motif chemokine CXCL4L1 regulate inflammation and thrombus formation
Cellular and Molecular Life Sciences (2022)
-
Label-free high-throughput screening assay for the identification of norepinephrine transporter (NET/SLC6A2) inhibitors
Scientific Reports (2021)
-
A study of the dopamine transporter using the TRACT assay, a novel in vitro tool for solute carrier drug discovery
Scientific Reports (2021)