Abstract
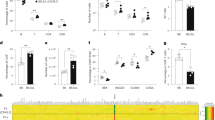
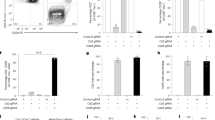
CCR5 is the major HIV-1 co-receptor, and individuals homozygous for a 32-bp deletion in CCR5 are resistant to infection by CCR5-tropic HIV-1. Using engineered zinc-finger nucleases (ZFNs), we disrupted CCR5 in human CD34+ hematopoietic stem/progenitor cells (HSPCs) at a mean frequency of 17% of the total alleles in a population. This procedure produces both mono- and bi-allelically disrupted cells. ZFN-treated HSPCs retained the ability to engraft NOD/SCID/IL2rγnull mice and gave rise to polyclonal multi-lineage progeny in which CCR5 was permanently disrupted. Control mice receiving untreated HSPCs and challenged with CCR5-tropic HIV-1 showed profound CD4+ T-cell loss. In contrast, mice transplanted with ZFN-modified HSPCs underwent rapid selection for CCR5−/− cells, had significantly lower HIV-1 levels and preserved human cells throughout their tissues. The demonstration that a minority of CCR5−/− HSPCs can populate an infected animal with HIV-1-resistant, CCR5−/− progeny supports the use of ZFN-modified autologous hematopoietic stem cells as a clinical approach to treating HIV-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
20 July 2010
In the version of this article initially published online, the callout to Figure 6b was written incorrectly as Figure 6c. Also, in Figure 2b, the label on the y axis was missing a “/” between CD4+ and CD8+. The errors have been corrected for the print, PDF and HTML versions of this article.
References
Wu, L. et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384, 179–183 (1996).
deRoda Husman, A.M., Blaak, H., Brouwer, M. & Schuitemaker, H. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J. Immunol. 163, 84597–84603 (1999).
Samson, M. et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996).
Novembre, J. et al. The geographic spread of the CCR5 Delta32 HIV-resistance allele. PLoS Biol. 3, e339 (2005).
Glass, W.G. et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J. Exp. Med. 203, 35–40 (2006).
Kantarci, O.H. et al. CCR5Δ32 polymorphism effects on CCR5 expression, patterns of immunopathology and disease course in multiple sclerosis. J. Neuroimmunol. 169, 137–143 (2005).
Rossol, M. et al. Negative association of the chemokine receptor CCR5 d32 polymorphism with systemic inflammatory response, extra-articular symptoms and joint erosion in rheumatoid arthritis. Arthritis Res. Ther. 11, R91–98 (2009).
Dau, B. & Holodiny, M. Novel targets for antiretroviral therapy: clinical progress to date. Drugs 69, 31–50 (2009).
Hutter, G. et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698 (2009).
Hutter, G., Schneider, T. & Thiel, E. Transplantation of selected or transgenic blood stem cells—a future treatment for HIV/AIDS? J. Int. AIDS Soc. 12, 10–14 (2009).
Anderson, J. et al. Safety and efficacy of a lentiviral vector containing three anti-HIV genes–CCR5 ribozyme, tat-rev siRNA, and TAR decoy–in SCID-hu mouse-derived T cells. Mol. Ther. 15, 1182–1188 (2007).
Bai, J. et al. Characterization of anti-CCR5 ribozyme-transduced CD34+ hematopoietic progenitor cells in vitro and in a SCID-hu mouse model in vivo. Mol. Ther. 1, 244–254 (2000).
Kumar, P. et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell 134, 577–586 (2008).
Swan, C.H. et al. T-cell protection and enrichment through lentiviral CCR5 intrabody gene delivery. Gene Ther. 13, 1480–1492 (2006).
Swan, C.H. & Torbett, B.E. Can gene delivery close the door to HIV-1 entry after escape? J. Med. Primatol. 35, 236–247 (2006).
Urnov, F.D. et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435, 646–651 (2005).
Jasin, M. et al. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 12, 224–228 (1996).
Sonoda, E. et al. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair (Amst.) 5, 1021–1029 (2006).
Perez, E.E. et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 26, 808–816 (2008).
Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106, 1565–1573 (2005).
Lombardo, A. et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 25, 1298–1306 (2007).
Hollis, R.P. et al. Stable gene transfer to human CD34(+) hematopoietic cells using the Sleeping Beauty transposon. Exp. Hematol. 34, 1333–1343 (2006).
Sumiyoshi, T. et al. Stable transgene expression in primitive human CD34+ hematopoietic stem/progenitor cells, using the Sleeping Beauty transposon system. Hum. Gene Ther. 20, 1607–1626 (2009).
Mátés, L. et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 41, 753–761 (2009).
Xue, X. et al. Stable gene transfer and expression in cord blood-derived CD34+ hematopoietic stem and progenitor cells by a hyperactive Sleeping Beauty transposon system. Blood 114, 1319–1330 (2009).
Basu, S. & Broxmeyer, H.E. CCR5 ligands modulate CXCL12-induced chemotaxis, adhesion, and Akt phosphorylation of human cord blood CD34+ cells. J. Immunol. 183, 7478–7488 (2009).
Watanabe, S. et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109, 212–218 (2007).
Brenchley, J.M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759 (2004).
Brenchley, J.M. et al. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7, 235–239 (2006).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003).
Talal, A.H. et al. Effect of HIV-1 infection on lymphocyte proliferation in gut-associated lymphoid tissue. J. Acquir. Immune Defic. Syndr. 26, 208–217 (2001).
Li, Q. et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434, 1148–1152 (2005).
Mattapallil, J.J. et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434, 1093–1097 (2005).
Veazey, R.S. et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998).
Berges, B.K. et al. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gamma c−/− (RAG-hu) mouse model. Retrovirology 3, 76–90 (2006).
Appay, V. & Sauce, D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol. 214, 231–241 (2008).
Stoddart, C.A. et al. IFN-alpha-induced upregulation of CCR5 leads to expanded HIV tropism in vivo. PLoS Pathog. 6, e1000766 (2010).
Choudhary, S.K. et al. R5 human immunodeficiency virus type 1 infection of fetal thymic organ culture induces cytokine and CCR5 expression. J. Virol. 79, 458–471 (2005).
Kahn, J.O. & Walker, B.D. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 339, 33–39 (1998).
Margolick, J.B. et al. Impact of inversion of the CD4/CD8 ratio on the natural history of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 42, 620–626 (2007).
Henrard, D.R. et al. Natural History of HIV-1 cell-free viremia. J. Am. Med. Assoc. 274, 554–558 (1995).
Chen, R.Y. et al. Distribution of health care expenditures for HIV-infected patients. Clin. Infect. Dis. 42, 1003–1010 (2006).
Richman, D.D. et al. The challenge of finding a cure for HIV infection. Science 323, 1304–1307 (2009).
Rossi, J.J., June, C.H. & Kohn, D.B. Genetic therapies against HIV. Nat. Biotechnol. 25, 1444–1454 (2007).
Bibikova, M. et al. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 161, 1169–1175 (2002).
Doyon, Y. et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 26, 702–708 (2008).
Santiago, Y. et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc. Natl. Acad. Sci. USA 105, 5809–5814 (2008).
Peters, W., Dupuis, M. & Charo, I.F. A mechanism for the impaired IFN-gamma production in C–C chemokine receptor 2 (CCR2) knockout mice: Role of CCR2 in linking the innate and adaptive immune responses. J. Immunol. 165, 7072–7077 (2000).
Smith, M.W. et al. CCR2 chemokine receptor and AIDS progression. Nat. Med. 3, 1052–1053 (1997).
Davis, B.R. & Candotti, F. Revertant somatic mosaicism in the Wiskott-Aldrich syndrome. Immunol. Res. 44, 127–131 (2009).
Hirschhorn, R. et al. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nat. Genet. 3, 290–295 (1996).
Hirschhorn, R. et al. In vivo reversion to normal of inherited mutations in humans. J. Med. Genet. 40, 721–728 (2003).
Stephan, V. et al. Atypical X-linked severe combined immunodeficiency due to possible spontaneous reversion of the genetic defect in T cells. N. Engl. J. Med. 335, 1563–1567 (1996).
Chun, T.W. et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis. 197, 714–720 (2008).
Lackner, A.A. et al. The gastrointestinal tract and AIDS pathogenesis. Gastroenterology 136, 1965–1978 (2009).
Picker, L.J. Immunopathogenesis of acute AIDS virus infection. Curr. Opin. Immunol. 18, 399–405 (2006).
Veazey, R.S., Marx, P.A. & Lackner, A.A. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22, 626–633 (2001).
Krishnan, A. et al. Autologous stem cell transplantation for HIV associated lymphoma. Blood 98, 3857–3859 (2001).
Aiuti, A. et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 296, 2410–2413 (2002).
Aiuti, A. et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 360, 447–458 (2009).
Ott, M.G. et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1–EVI1, PRDM16 or SETBP1. Nat. Med. 12, 401–409 (2006).
Cartier, N. et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326, 818–823 (2009).
Biti, R. et al. HIV-1 infection in an individual homozygous for the CCR5 deletion allele. Nat. Med. 3, 252–253 (1997).
Oh, D.Y. et al. CCR5Delta32 genotypes in a German HIV-1 seroconverter cohort and report of HIV-1 infection in a CCR5Delta32 homozygous individual. PLoS ONE 3, e2747–2753 (2008).
Weiser, B. et al. HIV-1 coreceptor usage and CXCR4-specific viral load predict clinical disease progression during combination antiretroviral therapy. AIDS 22, 469–479 (2008).
Ogert, R.A. et al. Mapping Resistance to the CCR5 co-receptor antagonist vicriviroc using heterologous chimeric HIV-1 envelope genes reveals key determinants in the C2–V5 domain of gp120. Virology 373, 387–399 (2008).
Soulie, C. et al. Primary genotypic resistance of HIV-1 to CCR5 antagonist treatment-naïve patients. AIDS 22, 2212–2214 (2008).
Palmer, S. et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 105, 3879–3884 (2008).
Dinoso, J.B. et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 106, 9403–9408 (2009).
Mitsuyasu, R.T. et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat. Med. 15, 285–292 (2009).
Shultz, L.D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hematopoietic stem cells. J. Immunol. 174, 6477–6489 (2005).
Rouet, F. et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J. Clin. Microbiol. 43, 2709–2717 (2005).
Sun, Z. et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204, 705–714 (2007).
Loken, M.R. et al. Establishing lymphocyte gates for immunophenotyping by flow cytometry. Cytometry 11, 453–459 (1990).
Acknowledgements
We would like to thank A. Cuddihy, S. Ge, R. Hollis and N. Smiley for expert technical assistance; C. Lutzko, V. Garcia, R. Akkina, B. Torbett and M. McCune for advice regarding humanized mice; and M. McCune for communicating unpublished data. This work was supported by funding from the California HIV/AIDS Research Project (P.M.C.), The Saban Research Institute (V.T.), and the National Heart, Lung, and Blood Institute P01 HL73104 (G.M.C., D.B.K. and P.M.C.).
Author information
Authors and Affiliations
Contributions
N.H. performed most of the experiments; J.W., K.K., G.F. and X.W. developed assays and analyzed samples; V.T. contributed to discussions; N.H., G.M.C., D.B.K., P.D.G., M.C.H. and P.M.C. designed the experiments and analyzed data; N.H. and P.M.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.W., K.K., G.F., P.D.G. and M.C.H. are employees of Sangamo BioSciences.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1,2 and Supplementary Figs. 1–4 (PDF 315 kb)
Rights and permissions
About this article
Cite this article
Holt, N., Wang, J., Kim, K. et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol 28, 839–847 (2010). https://doi.org/10.1038/nbt.1663
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1663
This article is cited by
-
Third patient free of HIV after receiving virus-resistant cells
Nature (2023)
-
Killing two birds with one stone: CRISPR/Cas9 CCR5 knockout hematopoietic stem cells transplantation to treat patients with HIV infection and hematological malignancies concurrently
Clinical and Experimental Medicine (2023)
-
HIV cure strategies: which ones are appropriate for Africa?
Cellular and Molecular Life Sciences (2022)
-
Recent advances in gene therapy: genetic bullets to the root of the problem
Clinical and Experimental Medicine (2022)
-
Editing out HIV: application of gene editing technology to achieve functional cure
Retrovirology (2021)