Abstract
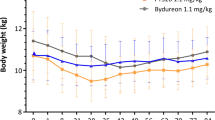
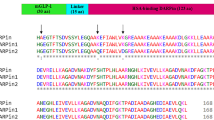
Increasing the in vivo residence times of protein therapeutics could decrease their dosing frequencies. We show that genetic fusion of an unstructured recombinant polypeptide of 864 amino acids, called XTEN, to a peptide or protein provides an apparently generic approach to extend plasma half-life. Allometric scaling suggests that a fusion of XTEN to the exenatide peptide should increase exenatide half-life in humans from 2.4 h to a projected time of 139 h. We confirmed the biological activity of the exenatide-XTEN fusion in mice. As extended stability might exacerbate undesirable side effects in some cases, we show that truncating the XTEN sequence can regulate plasma half-life. XTEN lacks hydrophobic amino acid residues that often contribute to immunogenicity and complicate manufacture. Based on data on XTEN fusions to exenatide, glucagon, GFP and human growth hormone, we expect that XTEN will enable dosing of otherwise rapidly cleared protein drugs at up to monthly intervals in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Kochendoerfer, G. Chemical and biological properties of polymer-modified proteins. Expert Opin. Biol. Ther. 3, 1253–1261 (2003).
Fishburn, C.S. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 97, 4167–4183 (2008).
Ryan, S.M., Mantovani, G., Wang, X., Haddleton, D.M. & Brayden, D.J. Advances in PEGylation of important biotech molecules: delivery aspects. Expert Opin. Drug Deliv. 5, 371–383 (2008).
Dhalluin, C. et al. Structural and biophysical characterization of the 40 kDa PEG-interferon-alpha2a and its individual positional isomers. Bioconjug. Chem. 16, 504–517 (2005).
Gaertner, H.F. & Offord, R.E. Site-specific attachment of functionalized poly(ethylene glycol) to the amino terminus of proteins. Bioconjug. Chem. 7, 38–44 (1996).
Shaunak, S. et al. Site-specific PEGylation of native disulfide bonds in therapeutic proteins. Nat. Chem. Biol. 2, 312–313 (2006).
Huang, L., Gough, P.C. & Defelippis, M.R. Characterization of poly(ethylene glycol) and PEGylated products by LC/MS with postcolumn addition of amines. Anal. Chem. 81, 567–577 (2009).
Bendele, A., Seely, J., Richey, C., Sennello, G. & Shopp, G. Short communication: renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicol. Sci. 42, 152–157 (1998).
Richter, A.W. & Akerblom, E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 70, 124–131 (1983).
Sroda, K. et al. Repeated injections of PEG-PE liposomes generate anti-PEG antibodies. Cell. Mol. Biol. Lett. 10, 37–47 (2005).
Sinigaglia, F. & Hammer, J. Motifs and supermotifs for MHC class II binding peptides. J. Exp. Med. 181, 449–451 (1995).
Alvarez, P., Buscaglia, C.A. & Campetella, O. Improving protein pharmacokinetics by genetic fusion to simple amino acid sequences. J. Biol. Chem. 279, 3375–3381 (2004).
Schlapschy, M. et al. Fusion of a recombinant antibody fragment with a homo-amino-acid polymer: effects on biophysical properties and prolonged plasma half-life. Protein Eng. Des. Sel. 20, 273–284 (2007).
Byetta package insert. (Amylin Pharmaceuticals, San Diego, 2009).
Drucker, D.J. et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372, 1240–1250 (2008).
Uversky, V.N. What does it mean to be natively unfolded? Eur. J. Biochem. 269, 2–12 (2002).
Cindric, M. et al. Structural characterization of PEGylated rHuG-CSF and location of PEG attachment sites. J. Pharm. Biomed. Anal. 44, 388–395 (2007).
ELSPAR package insert. (Merck & Co. Inc, Whitehouse Station, New Jersey, 2009).
Baggio, L.L., Huang, Q., Brown, T.J. & Drucker, D.J. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes 53, 2492–2500 (2004).
Glucagon package insert. (Eli Lilly & Co., Indianapolis, 2009).
Tucker, M. Very-low-dose glucagon may prevent nocturnal hypoglycemia. Pediatr. News 41, 58 (2007).
Harris, J.M. & Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 (2003).
He, X.H., Shaw, P.C. & Tam, S.C. Reducing the immunogenicity and improving the in vivo activity of trichosanthin by site-directed pegylation. Life Sci. 65, 355–368 (1999).
Shiffman, M.L. Pegylated interferons: what role will they play in the treatment of chronic hepatitis C? Curr. Gastroenterol. Rep. 3, 30–37 (2001).
Ai, G. et al. Single- and multiple-dose pharmacokinetics of exendin-4 in rhesus monkeys. Int. J. Pharm. 353, 56–64 (2008).
Chez, R.A. et al. Glucagon metabolism in nonhuman primate pregnancy. Am. J. Obstet. Gynecol. 120, 690–696 (1974).
Huang, Y. et al. Preparation and characterization of a novel exendin-4 human serum albumin fusion protein expressed in Pichia Pastoris. J. Pept. Sci. 14, 588–595 (2008).
Mahmood, I. Application of allometric principles for the prediction of pharmacokinetics in human and veterinary drug development. Adv. Drug Deliv. Rev. 59, 1177–1192 (2007).
Folta-Stogniew, E. & Williams, K.R. Determination of molecular masses of proteins in solution: Implementation of an HPLC size exclusion chromatography and laser light scattering service in a core laboratory. J. Biomol. Tech. 10, 51–63 (1999).
Acknowledgements
The size exclusion chromatography–multi-angle light scattering analysis was conducted by E. Folta-Stogniew at the W.M. Keck Foundation Biotechnology Resource Laboratory. The mass spectrometry was performed at MDS Analytical Technologies by A. Booy. The Macrocap Q resin was kindly provided by J. Lundberg from GE Healthcare. The project described was supported by award number R44GM079873 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
V.S., design of XTEN sequences and constructs, data analysis; C.-W.W., design of XTEN sequences and constructs; N.C.G., design of XTEN sequences and constructs, preparation/characterization of proteins, data analysis; B.J.S., design of XTEN sequences and constructs, preparation/characterization of proteins, data analysis; A.C., managing animal studies, animal sample analysis, data analysis; W.T., preparation/characterization of proteins; M.D.S., design of XTEN sequences and constructs; Y. Yin, design of XTEN sequences and constructs, preparation/characterization of proteins; Y. Yao, preparation/characterization of proteins; O.B., design of XTEN sequences and constructs; J.L.C., design of XTEN sequences and constructs, data analysis; J.S., design of XTEN sequences and constructs, data analysis; W.P.C.S., design of XTEN sequences and constructs, data analysis.
Corresponding author
Ethics declarations
Competing interests
V.S., C.-W. W., N.C.G., B.J.S., A.C., W.T., M.D.S., Y.Yin, Y.Yao, O.B. J.S. & W.P.C.S. are employees of Amunix. J.L.C. is an employee of Versatis.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–8 and Supplementary Tables 1–5 (PDF 2471 kb)
Rights and permissions
About this article
Cite this article
Schellenberger, V., Wang, Cw., Geething, N. et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat Biotechnol 27, 1186–1190 (2009). https://doi.org/10.1038/nbt.1588
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1588
This article is cited by
-
Derivation of a novel antimicrobial peptide from the Red Sea Brine Pools modified to enhance its anticancer activity against U2OS cells
BMC Biotechnology (2024)
-
Genetically Fused DARPins: A Novel Approach for Designing Extended-Release Thrombopoietin Mimetic Peptides
International Journal of Peptide Research and Therapeutics (2023)
-
Moonlighting chaperone activity of the enzyme PqsE contributes to RhlR-controlled virulence of Pseudomonas aeruginosa
Nature Communications (2022)
-
Site-directed RNA editing by harnessing ADARs: advances and challenges
Functional & Integrative Genomics (2022)
-
Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase
Nature Biotechnology (2020)