Abstract
Imaging human brain function with techniques such as magnetoencephalography1 typically requires a subject to perform tasks while their head remains still within a restrictive scanner. This artificial environment makes the technique inaccessible to many people, and limits the experimental questions that can be addressed. For example, it has been difficult to apply neuroimaging to investigation of the neural substrates of cognitive development in babies and children, or to study processes in adults that require unconstrained head movement (such as spatial navigation). Here we describe a magnetoencephalography system that can be worn like a helmet, allowing free and natural movement during scanning. This is possible owing to the integration of quantum sensors2,3, which do not rely on superconducting technology, with a system for nulling background magnetic fields. We demonstrate human electrophysiological measurement at millisecond resolution while subjects make natural movements, including head nodding, stretching, drinking and playing a ball game. Our results compare well to those of the current state-of-the-art, even when subjects make large head movements. The system opens up new possibilities for scanning any subject or patient group, with myriad applications such as characterization of the neurodevelopmental connectome, imaging subjects moving naturally in a virtual environment and investigating the pathophysiology of movement disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cohen, D. Magnetoencephalography: detection of the brain’s electrical activity with a superconducting magnetometer. Science 175, 664–666 (1972).
Kominis, I. K., Kornack, T. W., Allred, J. C. & Romalis, M. V. A subfemtotesla multichannel atomic magnetometer. Nature 422, 596–599 (2003).
Shah, V. K. & Wakai, R. T. A compact, high performance atomic magnetometer for biomedical applications. Phys. Med. Biol. 58, 8153–8161 (2013).
Hämäläinen, M. S., Hari, R., Ilmoniemi, R. J., Knuutila, J. & Lounasma, O. V. Magnetoencephalography: theory, instrumentation, and applications to non-invasive studies of the working human brain. Rev. Mod. Phys. 65, 413–497 (1993)
Gross, J. et al. Good practice for conducting and reporting MEG research. Neuroimage 65, 349–363 (2013).
Allred, J. C., Lyman, R. N., Kornack, T. W. & Romalis, M. V. High-sensitivity atomic magnetometer unaffected by spin-exchange relaxation. Phys. Rev. Lett. 89, 130801 (2002).
Shah, V., Knappe, S., Schwindt, P. D. D. & Kitching, J. Subpicotesla atomic magnetometry with a microfabricated vapour cell. Nat. Photon. 1, 649–652 (2007)
Dupont-Roc, J., Haroche, S. & Cohen-Tannoudji, C. Detection of very weak magnetic fields (10–9 gauss) by 87Rb zero-field level crossing resonances. Phys. Lett. A 28, 638–639 (1969)
Borna, A . et al. A 20-channel magnetoencephalography system based on optically pumped magnetometers. Phys. Med. Biol. 62, 8909–8923 (2017)
Alem, O., Benison, A. M., Barth, D. S., Kitching, J. & Knappe, S. Magnetoencephalography of epilepsy with a microfabricated atomic magnetrode. J. Neurosci. 34, 14324–14327 (2014).
Alem, O. et al. Magnetic field imaging with microfabricated optically-pumped magnetometers. Opt. Express 25, 7849–7858 (2017).
Boto, E. et al. A new generation of magnetoencephalography: room temperature measurements using optically-pumped magnetometers. Neuroimage 149, 404–414 (2017).
Johnson, C., Schwindt, P. D. D. & Weisend, M. Magnetoencephalography with a two-color pump-probe, fiber-coupled atomic magnetometer. Appl. Phys. Lett. 97, 243703 (2010)
Johnson, C. N., Schwindt, P. D. & Weisend, M. Multi-sensor magnetoencephalography with atomic magnetometers. Phys. Med. Biol. 58, 6065–6077 (2013).
Kamada, K. et al. Human magnetoencephalogram measurements using newly developed compact module of high-sensitivity atomic magnetometer. Jpn. J. Appl. Phys. 54, 026601 (2015)
Kim, K. et al. Multi-channel atomic magnetometer for magnetoencephalography: a configuration study. Neuroimage 89, 143–151 (2014).
Pfurtscheller, G. & Lopes da Silva, F. H. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 110, 1842–1857 (1999).
Gaetz, W., Macdonald, M., Cheyne, D. & Snead, O. C. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage 51, 792–807 (2010).
Mary, A. et al. Aging reduces experience-induced sensorimotor plasticity. a magnetoencephalographic study. Neuroimage 104, 59–68 (2015).
Robson, S. E. et al. Abnormal visuomotor processing in schizophrenia. Neuroimage Clin. 12, 869–878 (2016).
Uhlhaas, P. J. & Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 11, 100–113 (2010).
Barratt, E. L. et al. Abnormal task driven neural oscillations in multiple sclerosis: A visuomotor MEG study. Hum. Brain Mapp. 38, 2441–2453 (2017).
Brookes, M. J. et al. Optimising experimental design for MEG beamformer imaging. Neuroimage 39, 1788–1802 (2008).
Van Veen, B. D., van Drongelen, W., Yuchtman, M. & Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 44, 867–880 (1997)
Poole, M. & Bowtell, R. Novel gradient coils designed using a boundary element method. Concepts Magn. Reson. Part B Magn. Reson. Eng. 31, 162–175 (2007)
Carlson, J. W., Derby, K. A., Hawryszko, K. C. & Weideman, M. Design and evaluation of shielded gradient coils. Magn. Reson. Med. 26, 191–206 (1992).
Baillet, S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 20, 327–339 (2017).
Brookes, M. J. et al. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc. Natl Acad. Sci. USA 108, 16783–16788 (2011).
Hipp, J. F., Hawellek, D. J., Corbetta, M., Siegel, M. & Engel, A. K. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci. 15, 884–890 (2012).
Jerbi, K . et al. Coherent neural representation of hand speed in humans revealed by MEG imaging. Proc. Natl Acad. Sci. USA 104, 7676–7681 (2007)
Yoda, K. Analytical design method of shelf shielded planar coils. J. Appl. Phys. 67, 4349 (1990)
Vrba, J. & Robinson, S. E. Signal processing in magnetoencephalography. Methods 25, 249–271 (2001).
Robinson, S. & Vrba, J. in Recent Advances in Biomagnetism (eds Yoshimoto, T. et al.) 302–305 (Tohoku Univ. Press, 1998)
Backus, G. E. & Gilbert, F. The resolving power of gross Earth data. Geophys. J. R. Astron. Soc. 16, 169–205 (1968)
Sarvas, J. Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys. Med. Biol. 32, 11–22 (1987)
Acknowledgements
This study was funded by a Wellcome Collaborative Award in Science (203257/Z/16/Z and 203257/B/16/Z) awarded to G.R.B., R.B. and M.J.B. We also acknowledge the UK Quantum Technology Hub for Sensors and Metrology, funded by the Engineering and Physical Sciences Research Council (EP/M013294/1). We acknowledge Medical Research Council Grants (MR/K005464/1 and MR/M006301/1). The Wellcome Centre for Human Neuroimaging is supported by core funding from Wellcome (203147/Z/16/Z). OPM sensor development at QuSpin was supported by National Institutes of Health grants R44HD074495 and R44MH110288. The scanner-casts were designed and manufactured by M. Lim at Chalk Studios.
Author information
Authors and Affiliations
Contributions
E.B.: system design and fabrication, data collection, data analysis, data interpretation, writing paper. N.H.: system design and fabrication, data collection, data analysis, data interpretation, writing paper. J.L.: system design and fabrication, data collection, data analysis, data interpretation, writing paper. G.R.: system design and fabrication, data collection, data analysis, data interpretation, writing paper. V.S.: system design and fabrication. S.S.M.: data interpretation, writing paper. L.D.M.: data interpretation, writing paper. K.J.M.: data collection, data analysis, data interpretation, writing paper. T.M.T.: data interpretation, writing paper. S.B.: data collection, data interpretation, writing paper. G.R.B.: conceptualization, system design and fabrication, data interpretation, writing paper. R.B.: conceptualization, system design and fabrication, data collection, data analysis, data interpretation, writing paper. M.J.B.: conceptualization, system design and fabrication, data collection, data analysis, data interpretation, writing paper.
Corresponding author
Ethics declarations
Competing interests
V.S. is the founding director of QuSpin, the commercial entity selling OPM magnetometers. QuSpin built the sensors used here and advised on the system design and operation, but played no part in the subsequent measurements or data analysis. This work was funded by a Wellcome award which involves a collaboration agreement with QuSpin.
Additional information
Reviewer Information Nature thanks S. Baillet and R. Leahy for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
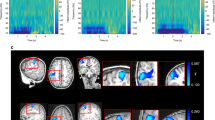
Extended Data Figure 1 Response magnitude as a function of subject movement.
a, Beta envelopes for finger abduction trials (blue/red) and resting trials (black/green) in the presence of large movement (red/green) and small movements (blue/black). b, The response size (that is, the difference between the mean amplitude during the desynchronization and rebound periods) shown as a function of maximum movement during a trial. Note that no measurable relationship was found. A significant (P = 0.0052, Pearson correlation) baseline shift was observed; this is likely to be a consequence of artefacts in the data generated by electrical activity in muscles controlling the naturalistic movements. See also Supplementary Information section 1.
Extended Data Figure 2 Quantification of spatial and temporal resolution.
a, A ‘seed’ location was selected in sensorimotor cortex (at the cross-hairs). Four thousand random ‘test’ locations (red squares), within 3 cm of the seed, were selected randomly and probed. Shared variance was measured between electrophysiological time courses at the seed and test locations. b, Left, correlation between the seed and test time courses plotted as a function of spatial separation. Right, source separation at which shared variance dropped to below 50%. In both cases the error bars show s.d. over test locations. This serves as an absolute quantification of spatial resolution. Note that the OPM array, when static, significantly outperforms SQUIDs (P = 0.002, Wilcoxon sum-rank test). c, Quantification of the robustness of the source orientation estimation. While source power can vary between experiments, source orientation relies only on the local orientation of the cortical sheet, and should therefore be the same across equivalent experiments. Here the histograms show the source orientation difference (as an angle) across runs for 4,000 locations of interest. Note for all three experiments (static OPMs (top), moving OPMs (middle) and SQUIDs (bottom)) the probability distribution peaks at zero as would be expected. The bar chart shows the probability of observing an angular discrepancy below 5°; note that the OPM array, when static, significantly (P < 0.05, permutation test) outperformed the SQUID array in terms of orientation robustness. Moving OPMs demonstrated the lowest robustness; however, this would be expected since the execution of natural movements differed across runs and therefore brain activity in the sensorimotor strip will also differ. The improvement in spatial specificity and robustness in our OPM-MEG system compared to a cryogenic (SQUID) system is likely to be a consequence of two factors: first, the closer proximity of the OPM sensors to the scalp provides higher signal-to-noise ratio in OPMs compared to SQUIDs; second, the scanner-cast ensures that, for each run, OPM sensors are in exactly the same location with respect to the brain. Cryogenic MEG, on the other hand, is subject to co-registration errors. d, Quantification of the OPM sensor’s frequency response, which also defines its temporal resolution. An OPM sensor was placed in a Helmholtz coil and a white noise current source applied to the coil. The blue line shows the Fourier spectrum of the current source, the green line shows the spectrum of the measured field. Note that sensitivity falls by 3 dB at 130 Hz, giving a temporal resolution of 7.7 ms. See also Supplementary Information section 2.
Extended Data Figure 3 Evoked response analysis.
a, Results when the subject was asked to remain still. b, Results when the subject was moving. Left panels show functional image: the overlay shows the spatial signature of the 2–30 Hz component of the evoked response, overlaid onto axial, sagittal and coronal slices of the anatomical MRI. Right panels show the time course of the evoked response; finger abduction trials in blue, rest trials in red. The shaded area shows standard error across six experiments. c, Direct comparison of the evoked response when the subject was asked to remain still (red) and was moving (blue). No significant SIR difference was found between static and moving runs (P = 0.24, two-sided Wilcoxon sum-rank test). See also Supplementary Information section 3.
Extended Data Figure 4 Gain changes with static magnetic field.
a, Raw OPM-MEG data recorded from a single channel during the gain experiment. Data were divided into nine segments (colour-coded here) corresponding to nine different static background magnetic fields ( ), ranging between −1.5 nT and 1.5 nT. The inset plot shows the small oscillating field (δBx), applied (in this sensor) at 137 Hz using the radially oriented on-sensor coil, which mimics neuromagnetic activity. b, Fourier transforms of each data segment. The inset figure shows the height of the 137-Hz peak for different segments. Note that the peak height changes as a function of static magnetic field. c, Fractional change in δBx as a function of background field
), ranging between −1.5 nT and 1.5 nT. The inset plot shows the small oscillating field (δBx), applied (in this sensor) at 137 Hz using the radially oriented on-sensor coil, which mimics neuromagnetic activity. b, Fourier transforms of each data segment. The inset figure shows the height of the 137-Hz peak for different segments. Note that the peak height changes as a function of static magnetic field. c, Fractional change in δBx as a function of background field  . The blue circles show the measured data with the standard deviation over the six sensors. The green line shows a fitted Lorentzian function. See also Supplementary Information section 4.
. The blue circles show the measured data with the standard deviation over the six sensors. The green line shows a fitted Lorentzian function. See also Supplementary Information section 4.
Extended Data Figure 5 Comparison of EEG and OPM-MEG.
a, Muscle tensing experiment. i, Channel montages for EEG (top) and OPM-MEG (bottom). Blue circles show EEG channels used; blue squares show MEG channels used; red stars denote channels used to create (ii); black circles indicate channels used for averages in (iii). ii, Time-frequency spectra showing fractional change in oscillatory amplitude, relative to baseline. The three plots show three separate channels, with the muscle artefact visible in the 0–1-s window, when jaw clenching took place. iii, Quantitative analysis of the magnitude of the artefact, which was measured to be about ten times larger in EEG. Error bars show s.d. across sensors. b, Finger abduction experiment. The four rows show OPM-MEG and EEG data with the subject stationary, followed by OPM-MEG and EEG data with the subject making natural movements. The left-hand column shows movement parameters. The left and left-centre time–frequency spectra show absolute difference from baseline of the MEG (in fT) and EEG (in μV) signals for individual channels, in the finger abduction and resting trials, respectively. These results have been averaged across all six experiments in both modalities. The right and right-centre time–frequency spectra show equivalent visualizations for a single representative experiment. Notice that, with the head stationary, MEG and EEG show similar results. However with the head moving, EEG data suffer from artefacts generated by muscle activity, to which the MEG data are less susceptible. See also Supplementary Information section 6.
Extended Data Figure 6 OPM-MEG derived functional connectivity.
A single subject took part in an experiment in which 5 min of OPM-MEG data were acquired in the resting state (subject was told to ‘think of nothing’). The experiment was repeated twice and the results averaged. a, A 26-channel OPM scalp array, with OPM sensors positioned (using a scanner-cast) approximately to cover the left and right parietal lobes (red circles). MEG data were reconstructed in source space using a beamformer, on a 4-mm grid covering the entire brain. A seed location was selected in left sensorimotor cortex and functional connectivity between the seed and the rest of the brain computed using an amplitude envelope correlation measurement, with correction for signal leakage by regression. b, Regions exhibiting the strongest functional connectivity to the seed location (in the beta frequency band). Note that, in addition to a region around the seed, functional connectivity is observed in the homologous regions of the opposite hemisphere. This reflects long-range functional connectivity within the sensorimotor network. c, Functional connectivity strength between left and right primary sensorimotor cortex, plotted as a function of frequency. Note that, as expected, functional connectivity between these regions is greatest in the beta band (13–30 Hz). d, An example of beta band envelopes from the left (blue) and right (red) sensorimotor cortices, derived from resting state data. See also Supplementary Information section 7.
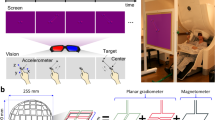
Extended Data Figure 7 An overview of the OPM-MEG system.
a, Schematic showing an overview of system hardware. b, Positioning of the reference sensors relative to the head to allow measurement of the three Cartesian components of the magnetic field, and the two dominant spatial gradients of the field. Each sensor provides measurements of two components of the magnetic field that are perpendicular to the beam axis. Both components were measured for field nulling, but during experimental measurements only the component of the field along the long-axis of the sensor was measured.
Extended Data Figure 8 Crosstalk characterization across an OPM array.
a, Schematic 3D representation of the crosstalk simulation. The head surface is shown with two example sensors. The locations of all 13 sensors are also indicated. We sought to characterize crosstalk between all pairs of sensors in the array. b, c, Simulated crosstalk between sensors measured as the ratio of fields generated by the perturbing sensor and the base sensor at the position of the base sensor. This ratio is a periodic function of sensor rotation about the radial orientation; the minimum interaction is shown in b, the maximum is shown in c. d, Experimentally measured crosstalk matrix. See also Supplementary Information section 8.
Extended Data Figure 9 Coil designs.
Wire paths and field plots are shown for the five coils: i, Bx; ii, By; iii, Bz; iv, dBx/dz; v, dBz/dz. The upper portion of each part shows the wire paths for one (1.6 × 1.6-m2) plane of the bi-planar coil. Red and blue colours indicate clockwise and anticlockwise circulation of the current. The lower portion shows contours of the field or field gradient strength over the 0.4 × 0.4-m2 x–y plane located at the centre of the volume of interest (z = 0). For v, contours are shown in the x–z plane at y = 0). The field or gradient values are normalized to the value at x = y = z = 0. Variation from the ideal field distribution is less than 5% over a 0.4 × 0.4 × 0.4-m3 central volume.
Extended Data Figure 10 Removal of muscle artefacts via beamforming.
a, The montage of OPM-MEG channels used to measure muscle artefact data. b, A beamformer image, highlighting a location of interest in right sensorimotor cortex. c, The time–frequency response for the best OPM-MEG sensor. d, Reconstructed responses from the over-regularized beamformer (which is analogous to dipole fitting) (left) and unregularized beamformer (right). Note that for unregularized beamforming the muscle artefact is supressed effectively. See also Supplementary Information section 9.
Supplementary information
Supplementary Information
This file contains: Trial-by-trial analysis of data; Spatiotemporal resolution and orientation robustness analysis; Evoked response analysis; Methods for OPM calibration to account for background fields; Discussion of the field nulling coil system; OPM MEG versus EEG comparison; Example applications in the measurement of brain connectivity; Simulation and experimental measurements of crosstalk between OPM sensors; Reduction of interference from muscle artifacts and Supplementary equipment schematics. (PDF 1319 kb)
Rights and permissions
About this article
Cite this article
Boto, E., Holmes, N., Leggett, J. et al. Moving magnetoencephalography towards real-world applications with a wearable system. Nature 555, 657–661 (2018). https://doi.org/10.1038/nature26147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature26147
This article is cited by
-
Using optically pumped magnetometers to replicate task-related responses in next generation magnetoencephalography
Scientific Reports (2024)
-
Long-baseline quantum sensor network as dark matter haloscope
Nature Communications (2024)
-
Coherence-based channel selection and Riemannian geometry features for magnetoencephalography decoding
Cognitive Neurodynamics (2024)
-
Combining OPM and lesion mapping data for epilepsy surgery planning: a simulation study
Scientific Reports (2024)
-
Neurophysiological alterations in mice and humans carrying mutations in APP and PSEN1 genes
Alzheimer's Research & Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.