Abstract
Acute pain represents a crucial alarm signal to protect us from injury1. Whereas the nociceptive neurons that convey pain signals were described more than a century ago2, the molecular sensors that detect noxious thermal or mechanical insults have yet to be fully identified3,4,5,6. Here we show that acute noxious heat sensing in mice depends on a triad of transient receptor potential (TRP) ion channels: TRPM3, TRPV1, and TRPA1. We found that robust somatosensory heat responsiveness at the cellular and behavioural levels is observed only if at least one of these TRP channels is functional. However, combined genetic or pharmacological elimination of all three channels largely and selectively prevents heat responses in both isolated sensory neurons and rapidly firing C and Aδ sensory nerve fibres that innervate the skin. Strikingly, Trpv1−/−Trpm3−/−Trpa1−/− triple knockout (TKO) mice lack the acute withdrawal response to noxious heat that is necessary to avoid burn injury, while showing normal nociceptive responses to cold or mechanical stimuli and a preserved preference for moderate temperatures. These findings indicate that the initiation of the acute heat-evoked pain response in sensory nerve endings relies on three functionally redundant TRP channels, representing a fault-tolerant mechanism to avoid burn injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Change history
02 May 2018
Please see accompanying Publisher correction (https://doi.org/10.1038/s41586-018-0100-8). In this Letter, the trace is missing in Fig. 1e. This error has been corrected online.
References
Grayson, M. Pain. Nature 535, S1 (2016)
Sherrington, C. S. The Integrative Action of the Nervous System (Oxford Univ. Press, 1906)
Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 29, 355–384 (2013)
Vriens, J ., Nilius, B. & Voets, T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 15, 573–589 (2014)
Geffeney, S. L. & Goodman, M. B. How we feel: ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron 74, 609–619 (2012)
Peirs, C. & Seal, R. P. Neural circuits for pain: recent advances and current views. Science 354, 578–584 (2016)
Caterina, M. J . et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997)
Pogorzala, L. A ., Mishra, S. K. & Hoon, M. A. The cellular code for mammalian thermosensation. J. Neurosci. 33, 5533–5541 (2013)
Binshtok, A. M ., Bean, B. P. & Woolf, C. J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449, 607–610 (2007)
Caterina, M. J . et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 (2000)
Davis, J. B . et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187 (2000)
Huang, S. M ., Li, X ., Yu, Y ., Wang, J. & Caterina, M. J. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain 7, 37 (2011)
Park, U . et al. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 31, 11425–11436 (2011)
Vriens, J . et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70, 482–494 (2011)
Cho, H . et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 15, 1015–1021 (2012)
Vriens, J . et al. Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nat. Chem. Biol. 10, 188–195 (2014)
Zimmermann, K . et al. Phenotyping sensory nerve endings in vitro in the mouse. Nat. Protocols 4, 174–196 (2009)
Everaerts, W . et al. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr. Biol. 21, 316–321 (2011)
Karashima, Y . et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl Acad. Sci. USA 106, 1273–1278 (2009)
Usoskin, D . et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015)
Tan, C. H. & McNaughton, P. A. The TRPM2 ion channel is required for sensitivity to warmth. Nature 536, 460–463 (2016)
Andersson, D. A ., Gentry, C ., Moss, S. & Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 28, 2485–2494 (2008)
Story, G. M . et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003)
Moparthi, L . et al. Human TRPA1 is a heat sensor displaying intrinsic U-shaped thermosensitivity. Sci. Rep. 6, 28763 (2016)
Arenas, O. M . et al. Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat. Neurosci. 20, 1686–1693 (2017)
Brenner, D. S ., Golden, J. P ., Vogt, S. K. & Gereau, R. W., IV . A simple and inexpensive method for determining cold sensitivity and adaptation in mice. J. Vis. Exp. (97): (2015). 10.3791/52640
Mishra, S. K ., Tisel, S. M ., Orestes, P ., Bhangoo, S. K. & Hoon, M. A. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 30, 582–593 (2011)
Caterina, M. J. & Pang, Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel) 9, e77 (2016)
Song, K . et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353, 1393–1398 (2016)
Tan, C. L . et al. Warm-sensitive neurons that control body temperature. Cell 167, 47–59.e15 (2016)
Acknowledgements
We thank all members of the Laboratories of Ion Channel Research and Experimental Gynecology and Obstetrics for comments and discussion, and K. Luyten for assistance with histological and in situ staining. This work was supported by grants from the KU Leuven Research Council (PF-TRPLe and C1-TRPLe to Tho.V. and R.V.), the Research Foundation-Flanders (FWO G.084515N to J.V. and Tho.V. and G.099114N to Tho.V. and Thi.V.), the Queen Elisabeth Medical Foundation for Neurosciences (to Tho.V.), the Belgian Foundation Against Cancer (to J.V. and Tho.V.) and the Planckaert-De Waele fund (to J.V.). K.D.C. and K.H. are holders of a doctoral fellowship of the FWO Belgium.
Author information
Authors and Affiliations
Contributions
I.V., M.M. and J.V. performed cellular calcium imaging experiments. I.V. designed, performed and analysed all skin nerve recordings, with technical input and guidance from K.Z. and A.S. K.H., J.V. and Tho.V. performed patch-clamp experiments. I.V., K.D.C, K.H., S.P., M.M. and J.V. performed behavioural experiments. K.D.C. and N.V.R. performed histological analyses. I.V., Thi.V. and A.S. performed and analysed RNA-seq experiments. S.P. generated DKO and TKO mice, with supervision by R.V. I.V. and Tho.V. wrote the manuscript with input from all co-authors. J.V. and Tho.V. initiated and supervised the entire project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks J. Wood and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Heat sensitivity is preserved in Trpm3−/−Trpv1−/− double knockout (DKOM3/V1) mice.
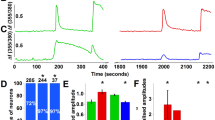
a, Percentages of wild-type (296 neurons from 6 mice) and DKOM3/V1 (1,353 neurons from 9 mice) trigeminal neurons responding to the indicated stimuli. b, Single-fibre nerve recording of a mechano- and heat-sensitive C fibre innervating the skin of a DKOM3/V1 mouse. c, Withdrawal latencies of wild-type (N = 10) and DKOM3/V1 (N = 7) mice in the tail-flick (at 57 °C) and hot-plate (at 50 °C) assays. Dotted lines indicate the cut-off time. Horizontal lines indicate mean. d, Venn diagram showing the level of functional coexpression of TRPM3, TRPV1 and TRPA1 in wild-type trigeminal neurons, based on responsiveness to PS, Caps and AITC (n = 662 neurons from 6 mice).
Extended Data Figure 2 TRPM8 expression and RNA-seq analysis of sensory neurons.
a, In situ hybridization using a TRPM8-specific probe in trigeminal ganglia of wild-type and TKO mice. b, Example of a sensory neuron from a TKO mouse responding to cold (15 °C) and the TRPM8 agonists menthol (50 μM) and icilin (1 μM). Scale bar, 6s/100 nM. c, Percentages of cold-responding and menthol-responding neurons in wild-type (n = 225 from 3 mice) and TKO mice (n = 189 from 3 mice). **P = 0.0089; Fisher’s exact test. d, Transcriptome-wide comparison of mRNA expression between wild-type and TKO mice. Several ion channels that have been previously implicated in heat sensing are indicated. e, Heat map showing the differential expression levels in single, double and triple knockout mice of a set of about 200 genes implicated in somatosensation20. Expression levels are defined as the log2 fold change compared to the wild type. Except for Trpm3, Trpv1 and Trpa1, no genes showed more than 50% up- or downregulation of expression in TKO compared to wild-type mice.
Extended Data Figure 3 Modulation of heat responses by H2O2: the role of TRPA1.
a, Representative examples of responses to heat, in the absence and presence of H2O2, in an AITC-sensitive and an AITC-insensitive sensory neuron from DKOM3/V1 mice. b, Example of the suppression of heat responses in an AITC-sensitive DKOM3/V1 neuron by the TRPA1 antagonist HC030031 (100 μM). c, Heat-insensitive TKO sensory neurons are also insensitive to H2O2 (n = 147 from 4 mice). d, e, Heat responses in TKO sensory neurons (which occur in only about 2% of the total population) are neither potentiated by H2O2 (400 μM; n = 147 from 4 mice) nor inhibited by 2-APB (250 μM; n = 150 from 4 mice). Scale bars in a–e, 60s/100 nM. f, Average heat-induced increases in intracellular calcium during the three consecutive heat responses in the protocol shown in a–d, for the indicated subtypes of neurons. Number of tested trigeminal neurons for the data in f: DKOM3/V1, n = 308 from 6 mice; DKOM3/V1 + HC030031, n = 104 from 4 mice; TKO, n = 147 from 4 mice. Group data are represented as mean ± s.e.m.
Extended Data Figure 4 H2O2 potentiates heat responses in CHO cells expressing mTRPA1.
a, Representative examples of responses to heat (45 °C), AITC (50 μM) and the ionophore ionomycin (2 μM) in CHO cells stably expressing mouse TRPA1. b, c, Potentiation of the heat response by H2O2 (100 μM) and inhibition by HC030031 (100 μM). d, Population analysis of all tested cells corresponding to the experiments in a–c. Control, n = 394; H2O2, n = 560; H2O2 + HC030031, n = 103 cells. As a conservative estimate of TRPA1-dependent heat responses, cells with a heat response larger than 5 s.d. above the mean of responses in the presence of HC030031 (dotted red line) were counted. Corresponding percentages are indicated. The H2O2 group was significantly different from the two other groups (***P < 0.00001; two-sided Mann–Whitney test with Bonferroni correction).
Extended Data Figure 5 Bimodal distribution of peak heat-induced firing rates in C fibres.
Histogram showing the peak firing rate of all tested heat-sensitive C fibres. Solid line shows the sum of two Gaussians (dotted lines) fit to the data. On the basis of these data, fibres were classified as either low frequency (LF; peak firing rate <23 Hz) or high frequency (HF; peak firing rate >23 Hz).
Extended Data Figure 6 Nerve bundles in the skin.
a, PGP9.5 staining of the plantar hind paw skin, representative of wild-type (N = 5) and TKO (N = 5) mice. Scale bar, 20 μm. Black arrows indicate nerve bundles. b, For each mouse, the nerve bundle areas of 20 sections were evaluated with an average distance of 0.1 mm between the cross sections. No significant difference was found between the two genotypes. P = 0.08; two-sided Student’s t-test. Group data are represented as mean ± s.e.m.
Extended Data Figure 7 Acute heat sensing at different temperatures.
a–d, Withdrawal latencies for male mice of different genotypes in the tail-immersion assay at the indicated temperatures. The cut-off (dashed line) was set at four times the mean withdrawal latency of wild-type mice. Number of tested animals: wild-type, N = 10; DKOM3/A1, N = 6; DKOV1/A1, N = 9; DKOM3/V1, N = 7; TKO, N = 9. Horizontal lines indicate means. e, P values for pair-wise comparisons of the withdrawal latencies of the different genotypes. A two-way ANOVA was performed with genotype and bath temperature as factors. P values represent the result of Tukey’s post-hoc tests for the factor genotype, and thus quantify global statistical differences in latency over the five tested temperatures (45, 48, 50, 52 and 57 °C). Similar results, yielding P < 10−4 for the comparison of TKO mice with either wild-type mice or mice of the different DKO genotypes, were obtained with a second independent cohort of male mice (5–6 mice per genotype).
Extended Data Figure 8 Acute heat sensing and burn injury.
a, Withdrawal latencies of female wild-type (N = 10) and TKO (N = 10) mice in the tail-immersion assay at the indicated temperatures. The cut-off (dashed line) was set at four times the mean withdrawal latency of wild-type mice. Indicated P values were obtained using a two-sided Student’s t-test. b, Withdrawal latencies of female wild-type (N = 10) and TKO (N = 10) mice in the hot-plate assay at the indicated temperatures. The cut-off (dashed line) was set at three times the mean withdrawal latency of wild-type mice. Indicated P values were obtained using two-sided Student’s t-tests. c, Sensitivity of female wild-type (N = 10) and TKO (N = 10) mice to calibrated von Frey hairs (P = 0.86; two-sided Student’s t-test). d, e, Photograph of the tails of male mice of the indicated genotypes (d) and close-up of the tails of five TKO mice (e) taken 3 days after a 57 °C tail-flick experiment. f, Percentage of mice of the indicated genotypes exhibiting visual signs of burn injury 3 days after a 57 °C tail-flick experiment (five mice per genotype).
Extended Data Figure 9 Thermal preference.
a, Preference index for the blue (<10 °C) versus the red zone (>45 °C) on the thermal gradient. Preference index was calculated as (tblue zone − tred zone)/(tblue zone + tred zone). ***P = 2.2 × 10−6, **P = 7.2 × 10−5, *P = 0.026, two-way ANOVA with Tukey’s post-hoc test for the factor genotype. Wild-type, N = 13; DKOM3/A1, N = 5; DKOV1/A1, N = 7; DKOM3/V1, N = 10; TKO, N = 10. b, Duration of the longest uninterrupted visit to the test plate. ***P = 7.7 × 10−5, **P = 0.0029, ##P = 0.0013, *P = 0.0072; one-way ANOVA with Tukey’s post-hoc test. c, Parameters describing thermal preference behaviour in the thermal gradient and two-plate preference tests. Number of tested animals: wild-type, N = 9; DKOM3/A1, N = 6; DKOV1/A1, N = 6; DKOM3/V1, N = 10; TKO, N = 9.
Supplementary information
Tail immersion assay of a WT and a TKO mouse.
Video showing the tail immersion assay at 57 °C in a wild type and a TKO mouse. (MP4 1538 kb)
Rights and permissions
About this article
Cite this article
Vandewauw, I., De Clercq, K., Mulier, M. et al. A TRP channel trio mediates acute noxious heat sensing. Nature 555, 662–666 (2018). https://doi.org/10.1038/nature26137
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature26137
This article is cited by
-
Thermal gradient ring for analysis of temperature-dependent behaviors involving TRP channels in mice
The Journal of Physiological Sciences (2024)
-
The kainate receptor GluK2 mediates cold sensing in mice
Nature Neuroscience (2024)
-
An ACC–VTA–ACC positive-feedback loop mediates the persistence of neuropathic pain and emotional consequences
Nature Neuroscience (2024)
-
Repetitive and compulsive behavior after Early-Life-Pain associated with reduced long-chain sphingolipid species
Cell & Bioscience (2023)
-
Involvement of skin TRPV3 in temperature detection regulated by TMEM79 in mice
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.