Abstract
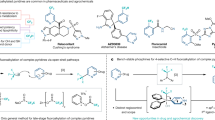
Aryl fluorides are widely used in the pharmaceutical and agrochemical industries1,2, and recent advances have enabled their synthesis through the conversion of various functional groups. However, there is a lack of general methods for direct aromatic carbon–hydrogen (C–H) fluorination3. Conventional methods require the use of either strong fluorinating reagents, which are often unselective and difficult to handle, such as elemental fluorine, or less reactive reagents that attack only the most activated arenes, which reduces the substrate scope. A method for the direct fluorination of aromatic C–H bonds could facilitate access to fluorinated derivatives of functional molecules that would otherwise be difficult to produce. For example, drug candidates with improved properties, such as increased metabolic stability or better blood–brain-barrier penetration, may become available. Here we describe an approach to catalysis and the resulting development of an undirected, palladium-catalysed method for aromatic C–H fluorination using mild electrophilic fluorinating reagents. The reaction involves a mode of catalysis that is unusual in aromatic C–H functionalization because no organometallic intermediate is formed; instead, a reactive transition-metal-fluoride electrophile is generated catalytically for the fluorination of arenes that do not otherwise react with mild fluorinating reagents. The scope and functional-group tolerance of this reaction could provide access to functional fluorinated molecules in pharmaceutical and agrochemical development that would otherwise not be readily accessible.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007)
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015)
Campbell, M. G. & Ritter, T. Modern carbon–fluorine bond forming reactions for aryl fluoride synthesis. Chem. Rev. 115, 612–633 (2015)
Sandford, G. Elemental fluorine in organic chemistry (1997–2006). J. Fluor. Chem. 128, 90–104 (2007)
Taylor, S. D., Kotoris, C. C. & Hum, G. Recent advances in electrophilic fluorination. Tetrahedron 55, 12431–12477 (1999)
Lal, G. S., Pez, G. P. & Syvret, R. G. Electrophilic NF fluorinating agents. Chem. Rev. 96, 1737–1756 (1996)
Fier, P. S. & Hartwig, J. F. Selective C–H fluorination of pyridines and diazines inspired by a classic amination reaction. Science 342, 956–960 (2013)
Chan, K. S. L., Wasa, M., Wang, X. & Yu, J.-Q. Palladium(ii)-catalyzed selective monofluorination of benzoic acids using a practical auxiliary: a weak-coordination approach. Angew. Chem. Int. Ed. 50, 9081–9084 (2011)
Wang, X., Mei, T.-S. & Yu, J.-Q. Versatile Pd(OTf)2·2H2O-catalyzed ortho-fluorination using NMP as a promoter. J. Am. Chem. Soc. 131, 7520–7521 (2009)
Hull, K. L., Anani, W. Q. & Sanford, M. S. Palladium-catalyzed fluorination of carbon–hydrogen bonds. J. Am. Chem. Soc. 128, 7134–7135 (2006)
Liu, W. et al. Oxidative aliphatic C–H fluorination with fluoride ion catalyzed by a manganese porphyrin. Science 337, 1322–1325 (2012)
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C–B bonds. Chem. Rev. 110, 890–931 (2010)
Cheng, C. & Hartwig, J. F. Rhodium-catalyzed intermolecular C–H silylation of arenes with high steric regiocontrol. Science 343, 853–857 (2014)
Wang, P. et al. Ligand-accelerated non-directed C–H functionalization of arenes. Nature 551, 489–493 (2017)
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J. & Glorius, F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew. Chem. Int. Ed. 51, 10236–10254 (2012)
Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 42, 8617–8636 (2013)
Regalado, E. L., Makarov, A. A., McClain, R., Przybyciel, M. & Welch, C. J. Search for improved fluorinated stationary phases for separation of fluorine-containing pharmaceuticals from their desfluoro analogs. J. Chromatogr. A 1380, 45–54 (2015)
Regalado, E. L. et al. Support of academic synthetic chemistry using separation technologies from the pharmaceutical industry. Org. Biomol. Chem. 12, 2161–2166 (2014)
Hyohdoh, I. et al. Fluorine scanning by nonselective fluorination: enhancing Raf/MEK inhibition while keeping physicochemical properties. ACS Med. Chem. Lett. 4, 1059–1063 (2013)
Cernak, T ., Dykstra, K. D ., Tyagarajan, S ., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016); erratum 46, 1760 (2017)
McCall, A. S. & Kraft, S. Pyridine-assisted chlorinations and oxidations by palladium(iv). Organometallics 31, 3527–3538 (2012)
McCall, A. S., Wang, H., Desper, J. M. & Kraft, S. Bis-N-heterocyclic carbene palladium(iv) tetrachloride complexes: synthesis, reactivity, and mechanisms of direct chlorinations and oxidations of organic substrates. J. Am. Chem. Soc. 133, 1832–1848 (2011)
Geng, C., Du, L., Liu, F., Zhu, R. & Liu, C. Theoretical study on the mechanism of selective fluorination of aromatic compounds with Selectfluor. RSC Adv. 5, 33385–33391 (2015)
Brandt, J. R., Lee, E., Boursalian, G. B. & Ritter, T. Mechanism of electrophilic fluorination with Pd(iv): fluoride capture and subsequent oxidative fluoride transfer. Chem. Sci. 5, 169–179 (2014)
Groves, J. T. High-valent iron in chemical and biological oxidations. J. Inorg. Biochem. 100, 434–447 (2006)
McNeill, E. & Du Bois, J. Ruthenium-catalyzed hydroxylation of unactivated tertiary C–H bonds. J. Am. Chem. Soc. 132, 10202–10204 (2010)
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007)
Liu, W. & Groves, J. T. Manganese catalyzed C–H halogenation. Acc. Chem. Res. 48, 1727–1735 (2015)
Du Bois, J. Rhodium-catalyzed C–H amination. An enabling method for chemical synthesis. Org. Process Res. Dev. 15, 758–762 (2011)
Boursalian, G. B., Ngai, M.-Y., Hojczyk, K. N. & Ritter, T. Pd-catalyzed aryl C–H imidation with arene as the limiting reagent. J. Am. Chem. Soc. 135, 13278–13281 (2013)
Paudyal, M. P. et al. Dirhodium-catalyzed C–H arene amination using hydroxylamines. Science 353, 1144–1147 (2016)
Acknowledgements
We thank L. Gitlin for HPLC purification, R. Goddard, S. Palm and J. Rust for X-ray crystallographic analysis, C. Farès for NMR spectroscopy, and M. Blumenthal, D. Kampen and S. Marcus for mass spectrometry. We thank UCB Biopharma for funding and compound separation, the Japan Society for the Promotion of Science and L’Oréal-UNESCO Japan for graduate fellowships to K.Y., the Fonds der Chemischen Industrie for a graduate fellowship for J.D.R., and the German Academic Exchange Service, DAAD for an Otto-Bayer fellowship to J.C.B.
Author information
Authors and Affiliations
Contributions
K.Y. designed catalyst 1 and optimized the fluorination reaction. K.Y. and J.D.R. conducted mechanistic studies. K.Y. and G.B.B. developed the conceptual approach to the project. J.L., J.A.O.G., J.C.B., K.Y. and J.D.R. explored the substrate scope. J.D.R. performed DFT calculations with input from M.v.G. and F.N. G.B.B. wrote the manuscript with input from all other authors. C.G. and J.J. supported development towards useful examples. K.Y., J.L., J.A.O.G., G.B.B., J.D.R. and T.R. analysed the data. T.R. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks J. Groves and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains procedures and characterization as well as spectra. (PDF 10929 kb)
Supplementary Data
This file contains checkcif 4na containing proof of appropriate x-ray data. (PDF 98 kb)
Supplementary Data
This file contains checkcif 4nb containing proof of appropriate x-ray data. (PDF 72 kb)
Rights and permissions
About this article
Cite this article
Yamamoto, K., Li, J., Garber, J. et al. Palladium-catalysed electrophilic aromatic C–H fluorination. Nature 554, 511–514 (2018). https://doi.org/10.1038/nature25749
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25749
This article is cited by
-
Contemporary synthetic strategies in organofluorine chemistry
Nature Reviews Methods Primers (2021)
-
Metal-organic layers as reusable solid fluorination reagents and heterogeneous catalysts for aromatic fluorination
Nano Research (2021)
-
Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination
Nature Chemistry (2020)
-
Direct C(sp2)–H alkylation of unactivated arenes enabled by photoinduced Pd catalysis
Nature Communications (2020)
-
α-Fluorination of carbonyls with nucleophilic fluorine
Nature Chemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.