Abstract
Animals must respond to various threats to survive. Neurons that express calcitonin gene-related peptide in the parabrachial nucleus (CGRPPBN neurons) relay sensory signals that contribute to satiation and pain-induced fear behaviour, but it is unclear how they encode these distinct processes. Here, by recording calcium transients in vivo from individual neurons in mice, we show that most CGRPPBN neurons are activated by noxious cutaneous (shock, heat, itch) and visceral stimuli (lipopolysaccharide). The same neurons are inhibited during feeding, but become activated during satiation, consistent with evidence that CGRPPBN neurons prevent overeating. CGRPPBN neurons are also activated during consumption of novel foods or by an auditory cue that has previously been paired with electrical footshocks. Correspondingly, silencing of CGRPPBN neurons attenuates the expression of food neophobia and conditioned fear responses. Therefore, in addition to transducing primary sensory danger signals, CGRPPBN neurons promote affective-behavioural states that limit harm in response to potential threats.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Saper, C. B. The house alarm. Cell Metab. 23, 754–755 (2016)
Saper, C. B. & Loewy, A. D. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 197, 291–317 (1980)
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013)
Campos, C. A., Bowen, A. J., Schwartz, M. W. & Palmiter, R. D. Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016)
Roman, C. W., Derkach, V. A. & Palmiter, R. D. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun. 7, 11905 (2016)
Ritter, R. C. Gastrointestinal mechanisms of satiation for food. Physiol. Behav. 81, 249–273 (2004)
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015)
Cai, H., Haubensak, W., Anthony, T. E. & Anderson, D. J. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 17, 1240–1248 (2014)
Lebow, M. A. & Chen, A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463 (2016)
Flusberg, B. A. et al. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat. Methods 5, 935–938 (2008)
Ghosh, K. K. et al. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878 (2011)
Bernard, J. F. & Besson, J. M. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J. Neurophysiol. 63, 473–490 (1990)
Bernard, J. F., Huang, G. F. & Besson, J. M. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J. Neurophysiol. 71, 1646–1660 (1994)
Ryan, P. J., Ross, S. I., Campos, C. A., Derkach, V. A. & Palmiter, R. D. Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat. Neurosci. 20, 1722–1733 (2017)
Braz, J., Solorzano, C., Wang, X. & Basbaum, A. I. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82, 522–536 (2014)
Liu, Q. et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365 (2009)
Schiavo, G., Matteoli, M. & Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80, 717–766 (2000)
Kim, J. C. et al. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron 63, 305–315 (2009)
Ranade, S. S. et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014)
Mu, D. et al. A central neural circuit for itch sensation. Science 357, 695–699 (2017)
Zhou, P . et al. Efficient and accurate extraction of in vivo calcium signals from microendoscope video data. eLife (in the press)
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015)
Reilly, S. & Trifunovic, R. Lateral parabrachial nucleus lesions in the rat: neophobia and conditioned taste aversion. Brain Res. Bull. 55, 359–366 (2001)
Carter, M. E., Han, S. & Palmiter, R. D. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J. Neurosci. 35, 4582–4586 (2015)
Tovote, P., Fadok, J. P. & Lüthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015)
Ricardo, J. A. & Koh, E. T. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 153, 1–26 (1978)
Day, H. E. W., Masini, C. V. & Campeau, S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 1025, 139–151 (2004)
Illing, R. B., Michler, S. A., Kraus, K. S. & Laszig, R. Transcription factor modulation and expression in the rat auditory brainstem following electrical intracochlear stimulation. Exp. Neurol. 175, 226–244 (2002)
Suzuki, T., Sugiyama, Y. & Yates, B. J. Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R965–R975 (2012)
Kaur, S. et al. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J. Neurosci. 33, 7627–7640 (2013)
Padilla, S. L. et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 19, 734–741 (2016)
Steculorum, S. M. et al. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165, 125–138 (2016)
Betley, J. N., Cao, Z. F., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013)
Kim, S. Y. et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013)
Kim, J., Zhang, X., Muralidhar, S., LeBlanc, S. A. & Tonegawa, S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479.e5 (2017)
Chen, Y., Lin, Y. C., Kuo, T. W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015)
Tokita, K., Inoue, T. & Boughter, J. D. Jr. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience 161, 475–488 (2009)
Zséli, G. et al. Elucidation of the anatomy of a satiety network: Focus on connectivity of the parabrachial nucleus in the adult rat. J. Comp. Neurol. 524, 2803–2827 (2016)
Myers, K. M. & Davis, M. Mechanisms of fear extinction. Mol. Psychiatry 12, 120–150 (2007)
Campos, C. A. et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci. 20, 934–942 (2017)
Resendez, S. L. et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat. Protocols 11, 566–597 (2016)
Acknowledgements
Research was supported by a fellowship grant from Hope Funds for Cancer Research (C.A.C.), a National Institutes of Health (NIH) training grant (C.W.R., T32DK007247), and an NIH grant (R.D.P., R01-DA24908). Inscopix provided the calcium imaging equipment via their DECODE program. We thank C. de Solages and L. Cardy (Inscopix) for advice regarding calcium imaging, Y. S. Jo for help with conditioning equipment, M. Chiang for maintaining the mouse colony, and S. Han for making the GCaMP6m virus.
Author information
Authors and Affiliations
Contributions
C.A.C. designed and conducted the calcium imaging studies. A.J.B. performed the stereotaxic surgeries for loss-of-function studies. C.A.C. and A.J.B. designed and conducted the fear studies. C.A.C. and C.W.R. designed and conducted the itch studies. R.D.P. provided guidance and resources. C.A.C. wrote the manuscript with input from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks C. Alexander and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 CGRPPBN neurons are activated by facial pain and electrical shock.
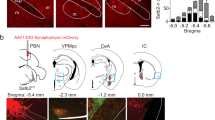
a, b, Percentage of cells activated by tail pinch and placing a warm metal rod on the lip of anaesthetized mice (n = 6 mice, 397 neurons). c, Representative calcium traces of the same individual CGRP neurons during baseline and 2 h after LPS injection in awake mice. d, Average calcium fluorescence activity 1 s before (Base) and during a footshock (0.5 mA, 0.5 s duration; n = 5 mice, 317 neurons); bar graph is mean ± s.e.m.. e, Calcium activity time course of CGRP neurons (n = 3 mice, 158 neurons) and oxytocin receptor neurons (n = 3 mice, 65 neurons) in response to electrical tail shock (2 mA, 2 s duration) in anaesthetized mice; baseline was 2-s period before shock. AAV-DIO-GCaMP6m was injected into the lateral PBN of CalcaCre/+ and OxtrCre/+ mice. For imaging oxytocin receptor neurons, the lens was placed over the dorsal lateral PBN, in contrast to external lateral PBN targeting for CGRP neurons. Line graph is mean ± s.e.m. (shaded region). ***P < 0.001, paired Student’s t-test (two-tailed). Related to Fig. 1. For statistical analysis, see Supplementary Information.
Extended Data Figure 2 CGRPPBN neurons encode stimulus intensity.
a, Plot shows calcium fluorescence activity of neurons in an anaesthetized mouse following placement of the tail in a water bath ranging from 40 °C to 52 °C (n = 56 neurons). b, Area under the curve of calcium activity following placement of tail in 40–52 °C water bath (n = 5 mice, 286 neurons). c, Percentage of cells activated by placement of tail in 56 or 60 °C water in anaesthetized mice; data points are individual mice (n = 4 mice) and line represents mean. d, Time course and peak neuronal responses to tail being placed in 56 or 60 °C water (n = 4 mice, 111 neurons). Baseline was 10-s period before placing tail in water. e, Area under the curve of calcium activity following placement of tail in water bath. f, Time course and peak neuronal responses to tail receiving graded intensities of electrical current (2–6 mA, 2 s duration; n = 3 mice, 105 neurons) in anaesthetized mice. Baseline was 2-s period before shock. g, Time course and peak calcium activity of neurons in an awake mouse (46 neurons) during exposure to a hotplate (40–56 °C, 20-s duration). Baseline was 2-min period in home cage before the hotplate test. h, Area under the curve of calcium activity following placement of awake mice on the hotplate (n = 3 mice, 126 neurons). Bar graphs are mean ± s.e.m.; line graphs are mean ± s.e.m. (shaded region). ***P < 0.001; dissimilar letters above columns indicate statistical differences between treatments. b, h, One-way repeated measures ANOVA, Tukey’s post-hoc; e, paired Student’s t-test (two-tailed). Related to Fig. 1. For statistical analysis, see Supplementary Information.
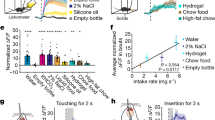
Extended Data Figure 3 Additional analysis of calcium imaging from fast–refeed study.
a, Average calcium fluorescence activity 5 s before and 5 s after presentation of standard chow pellet (n = 5 mice, 282 neurons); mean ± s.e.m.. b, Calcium activity relative to taking a bite (n = 5 mice, 286 neurons). c, d, Plot showing calcium activity (normalized ΔF/F) relative to placement of chow in cage (c) and relative to bite (d). Plots show neurons (rows) from all experimental mice (n = 5 mice, 282 neurons). e, f, Representative calcium traces extracted using CNMF analysis, relative to chow availability (e) and taking a bite (f). Βaseline was 60-s period before chow availability for all data shown. ***P < 0.001; dissimilar letters above columns indicate statistical differences between time points; a, paired Student’s t-test (two-tailed); c, one-way repeated measures ANOVA, Tukey’s post-hoc. Related to Fig. 3. For statistical analysis, see Supplementary Information.
Extended Data Figure 4 Additional analysis of calcium imaging from food neophobia studies.
a–c, Change in calcium fluorescence activity in response to a novel, high-fat diet (HFD) pellet. Test trials were conducted on consecutive days. Line graphs are means ± s.e.m. (shaded region) for raw ΔF/F values and plots show normalized ΔF/F values for neurons (rows) of all experimental mice. d–f, Average calcium activity 5 s before and 5 s after presentation of HFD pellet during three test trials. g–i, Average calcium activity 5 s before and 5 s after taking a bite from the HFD pellet. Bar graphs are means ± s.e.m. Test trial 1, n = 5 mice, 247 neurons; trial 2, n = 5 mice, 244 neurons; trial 3, n = 5 mice, 254 neurons. Βaseline was 60-s period before HFD pellet was presented. ***P < 0.001; d–i, paired Student’s t-test (two-tailed). Related to Fig. 4. For statistical analysis, see Supplementary Information.
Extended Data Figure 5 CGRPPBN neurons are activated during exposure to a novel marble.
a–c, Change in calcium activity in response to a marble being placed in home cage. Line graphs are means ± s.e.m. (shaded region) for raw ΔF/F values and plots show normalized ΔF/F values for neurons (rows) of all experimental mice. d–f, Average calcium activity 5 s before and 10 s after placing a marble in the cage. Graphs are means ± s.e.m. Test trial 1, n = 4 mice, 220 neurons; trial 2, n = 4 mice, 218 neurons; trial 3, n = 4 mice, 222 neurons. Βaseline was 60-s period before marble was presented. ***P < 0.001; d–f, paired Student’s t-test (two-tailed). Related to Fig. 4. See also Supplementary Video 4. For statistical analysis, see Supplementary Information.
Extended Data Figure 6 Additional calcium imaging analysis from fear recall studies.
a, b, Average calcium fluorescence activity during 10-s tone presentations in fear-conditioned (a) and sham-conditioned (b) mice during tone trials 1, 15, and 30. Relative fluorescence was calculated from a 5-s baseline period before tone presentation. Graphs are mean ± s.e.m.; fear-conditioned mice, n = 4 mice, 214 neurons; sham-conditioned mice, n = 4 mice, 169 neurons. c, Contextual fear conditioning paradigm in which mice were placed in a shock chamber before (Pre 1 and Pre 2) and after (Post 1 and Post 2) footshock conditioning. d, Calcium activity of individual neurons (horizontal lines are means) during a 5-min exposure to the shock chamber before and after fear conditioning (Pre 1, n = 3 mice, 120 neurons; Pre 2, n = 3 mice, 125 neurons; Post 1, n = 3 mice, 124 neurons; Post 2, n = 3 mice, 121 neurons). Relative fluorescence was normalized to a 2-min baseline recording period. e, Calcium activity of individual neurons (horizontal lines are means) during freezing and non-freezing behaviour after conditioning. f, Percentage of time spent freezing during exposure to the shock chamber after conditioning. Data points represent individual mice (n = 3). g, Representative individual neuron traces showing calcium activity while in home cage versus shock chamber after contextual fear conditioning. The dotted line denotes when the shock chamber recording began. The shaded regions annotate when the mouse was freezing. Dissimilar letters above columns indicate statistical differences between trials. ***P < 0.001; a, d, e, One-way repeated measures ANOVA, Tukey’s post-hoc. Related to Fig. 5. For statistical analysis, see Supplementary Information.
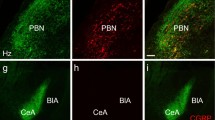
Extended Data Figure 7 Lens placement over CGRPPBN neurons.
Representative images of lens placement for imaging CGRP neurons (green). Dashed lines are approximations of lens placement. Scale bar is 100 μm.
Supplementary information
Supplementary Information
This file contains the detailed statistical analysis. (PDF 130 kb)
CGRPPBN neurons in anesthetized mice are activated by tail pinch
This is an unprocessed video file from calcium imaging. (MP4 5527 kb)
CGRPPBN neurons are inhibited before food consumption
Calcium recording was processed using CNMF, and the fluorescence of each neuron was normalized to its own maximum fluorescence intensity in the video. (MP4 7901 kb)
CGRPPBN neurons are active during exploration of novel, palatable food
Calcium recording was processed using CNMF, and the fluorescence of each neuron was normalized to its own maximum fluorescence intensity in the video. Video is 2x speed. (MP4 11980 kb)
CGRPPBN neurons are active during exploration of novel marble
Calcium recording was processed using CNMF, and the fluorescence of each neuron was normalized to its own maximum fluorescence intensity in the video. Video is 2x speed. (MP4 25197 kb)
Source data
Rights and permissions
About this article
Cite this article
Campos, C., Bowen, A., Roman, C. et al. Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622 (2018). https://doi.org/10.1038/nature25511
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25511
This article is cited by
-
Evidence for chronic headaches induced by pathological changes of myodural bridge complex
Scientific Reports (2024)
-
The parabrachial to central amygdala pathway is critical to injury-induced pain sensitization in mice
Neuropsychopharmacology (2024)
-
A spatially-resolved transcriptional atlas of the murine dorsal pons at single-cell resolution
Nature Communications (2024)
-
Excitatory neurons in the lateral parabrachial nucleus mediate the interruptive effect of inflammatory pain on a sustained attention task
Journal of Translational Medicine (2023)
-
Parabrachial tachykinin1-expressing neurons involved in state-dependent breathing control
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.