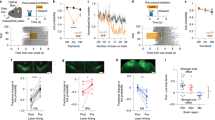
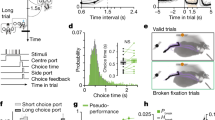
Abstract
Deciding when and whether to move is critical for survival. Loss of dopamine neurons (DANs) of the substantia nigra pars compacta (SNc) in patients with Parkinson’s disease causes deficits in movement initiation and slowness of movement1. The role of DANs in self-paced movement has mostly been attributed to their tonic activity, whereas phasic changes in DAN activity have been linked to reward prediction2,3. This model has recently been challenged by studies showing transient changes in DAN activity before or during self-paced movement initiation4,5,6,7. Nevertheless, the necessity of this activity for spontaneous movement initiation has not been demonstrated, nor has its relation to initiation versus ongoing movement been described. Here we show that a large proportion of SNc DANs, which did not overlap with reward-responsive DANs, transiently increased their activity before self-paced movement initiation in mice. This activity was not action-specific, and was related to the vigour of future movements. Inhibition of DANs when mice were immobile reduced the probability and vigour of future movements. Conversely, brief activation of DANs when mice were immobile increased the probability and vigour of future movements. Manipulations of dopamine activity after movement initiation did not affect ongoing movements. Similar findings were observed for the initiation and execution of learned action sequences. These findings causally implicate DAN activity before movement initiation in the probability and vigour of future movements.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jankovic, J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 79, 368–376 (2008)
Niv, Y., Daw, N. D. & Dayan, P. How fast to work: response vigor, motivation and tonic dopamine. Adv. Neural Inf. Process. Syst. 18, 1019–1026 (2005)
Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288 (2007)
Jin, X. & Costa, R. M. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature 466, 457–462 (2010)
Syed, E. C. J. et al. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nat. Neurosci. 19, 34–36 (2016)
Dodson, P. D. et al. Representation of spontaneous movement by dopaminergic neurons is cell-type selective and disrupted in Parkinsonism. Proc. Natl Acad. Sci. USA 113, E2180–E2188 (2016)
Howe, M. W. & Dombeck, D. A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510 (2016)
Gong, S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007)
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012)
Lima, S. Q., Hromádka, T., Znamenskiy, P. & Zador, A. M. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE 4, e6099 (2009)
Klaus, A. et al. The spatiotemporal organization of the striatum encodes action space. Neuron 95, 1171–1180 (2017)
Frey, B. J. & Dueck, D. Clustering by passing messages between data points. Science 315, 972–976 (2007)
Van Der Maaten, L. & Hinton, G. H. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008)
Mazzoni, P., Hristova, A. & Krakauer, J. W. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J. Neurosci. 27, 7105–7116 (2007)
Panigrahi, B. et al. Dopamine is required for the neural representation and control of movement vigor. Cell 162, 1418–1430 (2015)
Han, X. et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5, 18 (2011)
Barter, J. W. et al. Beyond reward prediction errors: the role of dopamine in movement kinematics. Front. Integr. Neurosci. 9, 39 (2015)
Hamid, A. A. et al. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 19, 117–126 (2016)
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013)
Atasoy, D., Aponte, Y., Su, H. H. & Sternson, S. M. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008)
Ghosh, K. K. et al. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878 (2011)
Wassum, K. M., Ostlund, S. B. & Maidment, N. T. Phasic mesolimbic dopamine signaling precedes and predicts performance of a self-initiated action sequence task. Biol. Psychiatry 71, 846–854 (2012)
Parker, N. F. et al. Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat. Neurosci. 19, 845–854 (2016)
Tecuapetla, F., Jin, X., Lima, S. Q. & Costa, R. M. Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166, 703–715 (2016)
Chuong, A. S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014)
Kravitz, A. V. et al. Regulation of Parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010)
Wong, A. L., Lindquist, M. A., Haith, A. M. & Krakauer, J. W. Explicit knowledge enhances motor vigor and performance: motivation versus practice in sequence tasks. J. Neurophysiol. 114, 219–232 (2015)
Thura, D. & Cisek, P. The basal ganglia do not select reach targets but control the urgency of commitment. Neuron 95, 1160–1170 (2017)
Spielewoy, C. et al. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav. Pharmacol. 11, 279–290 (2000)
Rosin, B. et al. Closed-loop deep brain stimulation is superior in ameliorating Parkinsonism. Neuron 72, 370–384 (2011)
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Academic, 2008)
Bäckman, C. M. et al. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 44, 383–390 (2006)
Sparta, D. R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2011)
Mathie, M. J., Lovell, N. H., Coster, A. C. F. & Celler, B. G. Determining activity using a triaxial accelerometer. In Proc. 2nd Joint EMBS-BMES Conf. 3, 2481–2482 (2002)
Cohen, J. Y., Haesler, S., Vong, L., Lowell, B. B. & Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88 (2012)
Thompson, K. G., Hanes, D. P., Bichot, N. P. & Schall, J. D. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J. Neurophysiol. 76, 4040–4055 (1996)
Thévenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998)
Zhou, P ., Resendez, S. L ., Stuber, G. D ., Kass, R. E. & Paninski, L. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. Preprint at https://arxiv.org/abs/1605.07266 (2016)
Pnevmatikakis, E. A. et al. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron 89, 285–299 (2016)
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011)
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007)
Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015)
Acknowledgements
We thank A. Vaz for mouse colony management, I. Vaz for the help during photoidentification experiments, L. Perry for help with stereological cell counts, A. Klaus, P. Zhou, L. Paninski for help with the application of the CNMF-E analysis, and the Champalimaud Hardware Platform (F. Carvalho, A. Silva, D. Bento) for help with the development of the motion sensors. This work was supported by fellowships from Gulbenkian Foundation to J.A.d.S. and Grants from Fundação para a Ciência e Tecnologia, Fronteras de la Ciencia-CONACyT-2022 and the IN226517 DGAPA-PAPIIT-UNAM to F.T. and from ERA-NET, European Research Council (COG 617142), and HHMI (IEC 55007415) to R.M.C.
Author information
Authors and Affiliations
Contributions
J.A.d.S. and R.M.C. designed the experiments and analyses and wrote the paper, J.A.d.S. performed all experiments and analyses, F.T. helped with optogenetic and recording experiments, V.P. helped with accelerometer experiments and accelerometer data analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks D. J. Surmeier and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Comparison between video-motion analysis and motion-sensor data in the open field.
a, Example of raw acceleration (static and dynamic acceleration) and angular velocity collected using a six-axis inertial sensor. b, Bivariate histogram of log pixel change and log acceleration in the open field (n = 13 sessions, from three mice). Notice the two clusters that emerge from this bivariate histogram (low acceleration and low pixel change cluster on the left, and high acceleration and high pixel change cluster on the right). The acceleration histogram provides a clear distinction between these two clusters. There is a high correlation between video-tracking measurements and acceleration (r = 0.74, n = 62,984 frames, P < 0.05). This also shows that animals rely on constant acceleration from their limbs to move, and that locomotion at a constant speed (acceleration 0) is highly unlikely. c, Heat maps of mean pixel change of video clips of 300 ms and 10 s (top and bottom, respectively), during which mice showed acceleration that was lower (left) or higher (right) than the threshold used to define immobility. d, Top, comparison between a video-derived motion measurement (pixel change) and total dynamic acceleration aligned to movement initiation determined using the acceleration threshold (n = 454 initiations obtained from three mice during a total of 13 open-field sessions). Bottom, representation of the movement of mice (based on the centre-of-mass) during each trial within the time intervals as indicated on the x axis. The trajectories were aligned to the centre-of-mass of the last frame of each 1-s interval. Different colours denote individual trials.
Extended Data Figure 2 TH-Cre line and ArchT infection characterization.
a, TH-Cre mice were crossed with ROSA26R-YFP mice (expression of YFP in Cre+ cells). This is an example of a midbrain slice of a TH-Cre × ROSA26R-YFP mouse with TH+ neurons labelled in red. The white line delimits the SNc, and the yellow and green lines delimit areas that cover a depth of 200 μm above and below the nigra, respectively, that were also targeted by stereological cell counts. Scale bar, 100 μm. b, Example of a SNc sampling field. Arrowheads denote examples of Cre+ cells that were TH+. Scale bars, 20 μm. c, Quantification of the specificity of the Cre line for tagging TH+ cells (n = 3 slices; 117 counting frames were analysed). d, Representative merged image of VTA and SNc after two weeks of infection. ArchT+ cells are labelled in green, TH+ cells are labelled in red and merged colours in yellow. ArchT+ cells are mainly confined to SNc. Scale bar, 500 μm. e, Detail of a SNc region labelled for TH (red) and ArchT (green) expression. Arrows are examples of TH+ and ArchT+ cells; closed arrowheads denote examples of TH+ and ArchT− cells. Scale bars, 20 μm. f, Efficiency of ArchT virus infection (left). Specificity of ArchT virus infection (right). This was calculated by quantifying the whole SNc stereologically, not only the area closest to the infection (n = 6 slices from two ArchT mice, 122 counting frames). g, Examples of the slices and fields used to do the stereological count shown in f and h. Scale bars, 100 μm. h, Stereological quantification of the number of SNc TH+ cells in YFP- and ArchT-expressing mice after two weeks of infection (ArchT, n = 6 slices from two ArchT mice, 122 counting frames; YFP, n = 6 slices from two YFP mice, 124 counting frames). i, Photomicrograph of a midbrain slice of a ArchT-expressing mouse at the end of the experiments (open field and FR8). Red indicates TH+ cells and green indicates ArchT+ cells. Scale bar, 100 μm. Data are mean ± s.e.m. (c, f, h).
Extended Data Figure 3 Photoidentification and clustering of SNc dopamine neurons.
a, Photomicrograph of a midbrain slice of a TH-Cre;Ai32 mouse denoting the right SNc and VTA. ChR2 in green and TH+ cells in red. Initial electrode position (dashed square) and distance travelled (dashed triangle). Scale bar, 100 μm. b, Example of continuous recording of a photoidentified neuron. Blue triangles denote 10-ms light pulses of blue light that were delivered at 1 Hz. c, PETH of the neuron in b aligned to blue-light delivery (100 pulses). d, Histogram of latencies to modulation by light delivery. A threshold of 7 ms and an increase in at least 30% firing rate was used to define neurons as photoidentified (blue bars). e, Mean spike traces for all photoidentified neurons used in Fig. 2. The black trace represents the mean of spikes obtained without light delivery and the blue trace represents the mean trace of spikes obtained during light delivery. f, Left, the area under the ROC curve (auROC) was calculated for each time bin of each significantly modulated neuron. Right, we used an affinity propagation algorithm to cluster the traces that resulted from the auROC analysis (see Methods for details). Four clusters were found, of which the PETH of the representative neuron is shown. Neurons were: transiently active before the initiation of movement (blue), transiently active before the initiation of movement followed by inhibition after the initiation (grey), sustained increase in activity with movement initiation (green) or negatively modulated (red).
Extended Data Figure 4 Calcium imaging of TH+ neurons from the SNc during movement initiation.
a, Example of the average pixel per pixel ΔF of one mouse aligned to movement initiation (n = 46 trials). Black arrowheads denote three neurons that are significantly activated before movement initiation. b, Proportion of neurons that were positively or negatively modulated and not modulated by movement initiation (n = 22 neurons obtained from three mice). c, Mean auROC trace of positively modulated neurons based on calcium imaging (solid line, n = 7). d, Mean auROC trace of negatively modulated neurons according to calcium imaging (solid line, n = 4). Grey shadow denotes s.e.m. (c, d).
Extended Data Figure 5 In vivo external recordings reveal specific inhibition of neuronal activity in the SNc.
a, Mean unit activity aligned to light onset (values less than 0.5 auROC indicate a decrease compared to baseline and more than 0.5 auROC indicate an increase compared to baseline) at different recording depths. The green rectangle signals the duration of light delivery. Left, mean of all units recorded. Right, mean of all units except negatively modulated units. b, Top, anatomical representation31 of the mean unit activity depending on recording depth and the location of the cannula of the recording electrode (red, decrease from baseline; blue, increase from baseline). The percentage of inhibited cells was not homogeneous throughout all depths (χ24,140 = 18.01, P < 0.05, test based on five levels of depth from –3.9 to –4.6 mm with 150-μm steps). In fact, when we investigated the mean activity of all units recorded at each depth, we found that the mean activity during light delivery changed depending on the depth, and it was only significantly different at the depths at which the SNc is located, for which the percentage of inhibited units was 61.3%. This is anatomically represented in b. Depth (number of neurons): −3.9 mm (3); −4 mm (22); −4.1 mm (14); −4.2 mm (6); −4.3 mm (8); −4.4 mm (10); −4.5 mm (4); −4.6 mm (5). Kruskal–Wallis test: H = 18.22; P = 0.011. Dunn’s multiple comparison test, all means compared to mean at −4.6 mm: 3.9 mm, P > 0.99; −4 mm, P = 0.078; −4.1 mm, *P = 0.017; −4.2 mm, **P = 0.008; −4.3 mm, P = 0.67; −4.4 mm, P = 0.82; −4.5 mm, P > 0.99). Asterisks indicate depths with mean auROC significantly different from −4.6 mm depth. c, Example of a single unit inhibited by green light. The mouse brain has been reproduced with permission from ref. 31.
Extended Data Figure 6 Five-second inhibition of SNc TH+ neurons during mobile and immobile trials.
a, Acceleration during laser-off and brief laser-on trials (5 s inhibition) when ArchT mice were mobile before the start of the trial (n = 217 laser-on trials and n = 212 laser-off trials obtained from 11 ArchT mice). The horizontal dotted line denotes the threshold used to classify acceleration state. b, Acceleration during laser-off and brief laser-on trials (5 s inhibition) when ArchT mice were immobile before trial start (n = 17 laser-on trials from 5 ArchT mice and n = 12 laser-off trials obtained from 7 ArchT mice). Horizontal dotted line denotes the threshold used to classify acceleration state. c, Acceleration during brief laser-on (5 s inhibition) normalized to mean laser-off acceleration for immobile and mobile states (n = 17 laser-on immobile trials obtained from 5 ArchT mice, n = 12 laser-off immobile trials obtained from 7 ArchT mice; n = 217 laser-on mobile trials, n = 212 laser-off mobile trials obtained from 11 mice). Acceleration state significantly affected normalized acceleration (linear mixed model with ‘mouse’ as a random effect, F = 19.57, P < 0.0001).
Extended Data Figure 7 Anatomical position of fibres, electrodes and lens.
a, Fibre placement (green, ArchT mice; yellow, YFP mice). b, Fibre placement (blue, ChR2 mice; yellow, YFP mice). c, Optrode placement. Horizontal blue lines denotes the cannula position and vertical lines the distance travelled by the electrodes. d, GRIN lens placement (green bar indicates the position of the bottom of the lens). The representations of the mouse brain and anatomical structures was obtained from the Allen Mouse Brain Atlas (2004) using API. Top, http://api.brain-map.org/api/v2/svg_download/100960073?groups=28; middle, http://api.brain-map.org/api/v2/svg_download/100960057?groups=28; bottom, http://api.brain-map.org/api/v2/svg_download/100960525?groups=28.
Extended Data Figure 8 Lever-press significant neurons across training days.
a, Field-of view of one of the GCamP6-expressing mice across different training days spanning days from the beginning to the end of training. Scale bars, 100 μm. b, Percentage of neurons significantly modulated by the sequence lever presses throughout training. Data shown were obtained during the training of three out of the four mice used to obtain the calcium imaging data shown in Fig. 4. Data during training was not available for one mouse.
Extended Data Figure 9 First press- and reward-related neurons populations do not overlap.
a, Example of a neuron modulated by first press but not reward (left) and a neuron related to reward but not first press (right), aligned to first press (blue) and reward consumption (red). b, Monte Carlo simulations (10,000 samples) were used to generate a distribution of the number of overlapping neurons for first press and reward, assuming random assignment. Red lines denote the 95% confidence interval. Dashed line represents the number of overlapping neurons found in our experiment.
Extended Data Figure 10 FR8-inhibition experiment using Jaws.
We replicated the result obtained in the FR8 task (Fig. 4h–j), using Jaws (see Methods for details). a, Latency to initiate lever press sequence for laser-off trails and trials with inhibition starting just before sequence initiation for both Jaws (n = 6) and GFP (n = 6) groups. Two-way mixed ANOVA; planned comparisons between laser-on and laser-off trials using Fisher’s least significant difference tests; main effect group F1,10 = 3.16, P = 0.11; main effect laser F1,10 = 9.074, P = 0.0131; interaction effect F1,10 = 0.92, P = 0.36; planned comparisons: Jaws laser off − laser on P = 0.019, GFP laser off − laser on P = 0.18. b, Press rate in trials with no light delivery and trials with light delivery starting after the first press for both Jaws (n = 5) and GFP (n = 6) groups. Two-way mixed ANOVA; planned comparisons between laser-on and laser-off trials using Fisher’s least significant difference tests; main effect group F1,9 = 1.607, P = 0.24; main effect laser F1,9 = 0.53, P = 0.49; interaction effect F1,9 = 1.01, P = 0.34; planned comparisons: Jaws laser off − laser on P = 0.86, GFP laser off − laser on P = 0.23.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1. (PDF 144 kb)
Rights and permissions
About this article
Cite this article
da Silva, J., Tecuapetla, F., Paixão, V. et al. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248 (2018). https://doi.org/10.1038/nature25457
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25457
This article is cited by
-
Neural inhibition as implemented by an actor-critic model involves the human dorsal striatum and ventral tegmental area
Scientific Reports (2024)
-
Dynamic behaviour restructuring mediates dopamine-dependent credit assignment
Nature (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
The cerebellum directly modulates the substantia nigra dopaminergic activity
Nature Neuroscience (2024)
-
Free will: An Example of the Dopaminergic System
Integrative Psychological and Behavioral Science (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.