Abstract
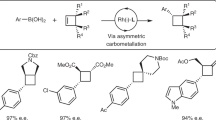
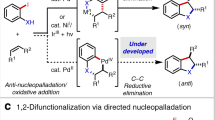
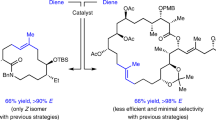
Catalytic cross-metathesis is a central transformation in chemistry, yet corresponding methods for the stereoselective generation of acyclic trisubstituted alkenes in either the E or the Z isomeric forms are not known. The key problems are a lack of chemoselectivity—namely, the preponderance of side reactions involving only the less hindered starting alkene, resulting in homo-metathesis by-products—and the formation of short-lived methylidene complexes. By contrast, in catalytic cross-coupling, substrates are more distinct and homocoupling is less of a problem. Here we show that through cross-metathesis reactions involving E- or Z-trisubstituted alkenes, which are easily prepared from commercially available starting materials by cross-coupling reactions, many desirable and otherwise difficult-to-access linear E- or Z-trisubstituted alkenes can be synthesized efficiently and in exceptional stereoisomeric purity (up to 98 per cent E or 95 per cent Z). The utility of the strategy is demonstrated by the concise stereoselective syntheses of biologically active compounds, such as the antifungal indiacen B and the anti-inflammatory coibacin D.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Negishi, E. et al. Recent advances in efficient and selective synthesis of di-, tri-, and tetrasubstituted alkenes via Pd-catalyzed alkenylation–carbonyl olefination synergy. Acc. Chem. Res. 41, 1474–1485 (2008)
Siau, W.-Y., Zhang, Y. & Zhao, Y. Stereoselective synthesis of Z-alkenes. Top. Curr. Chem. 327, 33–58 (2012)
Shang, G., Li, W. & Zhang, X. in Catalytic Asymmetric Synthesis (ed. Ojima, I. ) 344–436 (Wiley, 2010)
Baslé, O., Denicourt-Nowicki, A., Crévisy, C. & Mauduit, M. in Copper-Catalyzed Asymmetric Synthesis (eds Alexakis, A., Krause, N. & Woodward, S. ) 85–119 (VCH–Wiley, 2014)
Alexakis, A., Krause, N. & Woodward, S. in Copper-Catalyzed Asymmetric Synthesis (eds Alexakis, A., Krause, N. & Woodward, S. ) 33–68 (VCH–Wiley, 2014)
Sreekumar, C., Darst, K. P. & Still, W. C. A direct synthesis of Z-trisubstituted allylic alcohols via the Wittig reaction. J. Org. Chem. 45, 4260–4262 (1980)
Maryanoff, B. E. & Reitz, A. B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 89, 863–927 (1989)
Taber, D. F., Meagley, R. P. & Doren, D. J. Cyclohexenone construction by intramolecular alkylidene C–H insertion: Synthesis of (+)-cassiol. J. Org. Chem. 61, 5723–5728 (1996)
Trost, B. M. & Ball, Z. T. Addition of metalloid hydrides to alkynes: hydrometallation with boron, silicon, and tin. Synthesis 2005, 853–887 (2005)
Wang, C., Tobrman, T., Xu, Z. & Negishi, E. Highly regio- and stereoselective synthesis of (Z)-trisubstituted alkenes via propyne bromoboration and tandem Pd-catalyzed cross-coupling. Org. Lett. 11, 4092–4095 (2009)
Minato, A. & Suzuki, K. A remarkable steric effect in palladium-catalyzed Grignard coupling: regio- and stereoselective monoalkylation and -arylation of 1,1-dichloro-1-alkenes. J. Am. Chem. Soc. 109, 1257–1258 (1987)
Mun, B., Kim, S., Yoon, H., Kim, K. H. & Lee, Y. Total synthesis of isohericerin, isohericenone, and erinacerin A: development of a copper-catalyzed methylboronation of terminal alkynes. J. Org. Chem. 82, 6349–6357 (2017)
Negishi, E., Van Horn, D. E. & Yoshida, T. Controlled carbometallation. 20. Carbometalation reaction of alkynes with organoalane–zirconocene derivatives as a route to stereo- and regiodefined trisubstituted alkenes. J. Am. Chem. Soc. 107, 6639–6647 (1985)
Fleming, I., Newton, T. W. & Roessler, F. The silylcupration of acetylenes: A synthesis of vinylsilanes. J. Chem. Soc. Perkin Trans. I 2527–2532 (1981)
Ma, S. & Negishi, E. Anti-carbometalation of homopropargyl alcohols and their higher homologues via non-chelation-controlled syn-carbometallation and chelation-controlled isomerization. J. Org. Chem. 62, 784–785 (1997)
Lu, Z. & Ma, S. Studies on the Cu(I)-catalyzed regioselective anti-carbometallation of secondary terminal propargylic alcohols. J. Org. Chem. 71, 2655–2660 (2006)
Gribble, G. W. Biological activity of recently discovered halogenated marine natural products. Mar. Drugs 13, 4044–4136 (2015)
Chatterjee, A. K. & Grubbs, R. H. Synthesis of trisubstituted alkenes via olefin cross-metathesis. Org. Lett. 1, 1751–1753 (1999)
Chatterjee, A. K., Sanders, D. P. & Grubbs, R. H. Synthesis of symmetrical trisubstituted olefins by cross metathesis. Org. Lett. 4, 1939–1942 (2002)
Morrill, C. M., Funk, T. W. & Grubbs, R. H. Synthesis of tri-substituted vinyl boronates via ruthenium-catalyzed olefin cross-metathesis. Tetrahedr. Lett. 45, 7733–7736 (2004)
Wang, Z. J., Jackson, W. R. & Robinson, A. J. An efficient protocol for the cross-metathesis of sterically demanding olefins. Org. Lett. 15, 3006–3009 (2013)
Hoveyda, A. H., Khan, R. K. M., Torker, S. & Malcolmson, S. J. in Handbook of Metathesis (eds Grubbs, R. H., Wenzel, A. G., O’Leary, D. J. & Khosravi, E. ) 503–562 (Wiley–VCH, 2014)
Hoveyda, A. H. Evolution of catalytic stereoselective olefin metathesis: from ancillary transformation to purveyor of stereochemical identity. J. Org. Chem. 79, 4763–4792 (2014)
Hoveyda, A. H. & Zhugralin, A. R. The remarkable metal-catalysed olefin metathesis reaction. Nature 450, 243–251 (2007)
Cuvigny, T., du Penhoat, H. & Julia, M. Isomérisation cis trans régiosélective de doubles liaison trisubstitutées. Tetrahedr. Lett. 21, 1331–1334 (1980)
Schrock, R. R. & Hoveyda, A. H. Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts. Angew. Chem. Int. Ed. 42, 4592–4633 (2003)
Nguyen, T. T. et al. Kinetically controlled E-selective catalytic olefin metathesis. Science 352, 569–575 (2016)
Koh, M. J., Nguyen, T. T., Zhang, H., Schrock, R. R. & Hoveyda, A. H. Direct synthesis of Z-alkenyl halides through catalytic cross-metathesis. Nature 531, 459–465 (2016)
Lam, J. K. et al. Synthesis and evaluation of molybdenum and tungsten monoaryloxide halide alkylidene complexes for Z-selective cross-metathesis of cyclooctene and Z-1,2-dichloroethylene. J. Am. Chem. Soc. 138, 15774–15783 (2016)
Chemler, S. R., Trauner, D. & Danishefsky, S. J. The B-alkyl Suzuki–Miyaura cross-coupling reaction: Development, mechanistic study, and applications in natural product synthesis. Angew. Chem. Int. Ed. 40, 4544–4568 (2001)
Miyazawa, M., Ishibashi, N., Ohnuma, S. & Miyashita, M. Stereospecific internal alkylation of terminal γ,δ-epoxy acrylates. Tetrahedr. Lett. 38, 3419–3422 (1997)
Anantoju, K. K., Mohd, B. S. & Maringanti, T. C. An efficient and concise synthesis of indiacen A and indiacen B. Tetrahedr. Lett. 58, 1499–1500 (2017)
Ely, R. J. & Morken, J. P. Stereoselective nickel-catalyzed 1,4-hydroboration of 1,3-dienes. Org. Synth. 88, 342–352 (2011)
Mori, A., Fujiwara, J., Maruoka, K. & Yamamoto, H. Nucleophilic cleavage of acetals using organometallic reagents. J. Organomet. Chem. 285, 83–94 (1985)
Brown, H. C., Bhat, N. G. & Rajagopalan, S. Stereoselective synthesis of (E)- and (Z)-disubstituted vinyl bromides via organoboranes. Synthesis 480–482 (1986)
Kirchhoff, J. H., Netherton, M. R., Hills, I. D. & Fu, G. C. Boronic acids: New coupling partners in room-temperature Suzuki reactions of alkyl bromides. Crystallographic characterization of an oxidative-addition adduct generated under remarkably mild conditions. J. Am. Chem. Soc. 124, 13662–13663 (2002)
Zhang, H., Yu, E. C., Torker, S., Schrock, R. R. & Hoveyda, A. H. Preparation of macrocyclic Z-enoates and (E,Z)- or (Z,E)-dienoates through catalytic stereoselective ring-closing metathesis. J. Am. Chem. Soc. 136, 16493–16496 (2014)
Koh, M. J. et al. Molybdenum chloride catalysts for Z-selective olefin metathesis reactions. Nature 542, 80–85 (2017)
Edwards, J. T. et al. Decarboxylative alkenylation. Nature 545, 213–218 (2017)
Steinmetz, H. et al. Indiacens A and B: prenyl indoles from the myxobacterium Sandaracinus amylolyticus. J. Nat. Prod. 75, 1803–1805 (2012)
Marsch, N., Jones, P. G. & Lindel, T. SmI2-mediated dimerization of indolylbutenones and synthesis of the myxobacterial natural product indiacen B. Beilstein J. Org. Chem. 11, 1700–1706 (2015)
Coombs, J. R., Zhang, L. & Morken, J. P. Synthesis of vinyl boronates from aldehydes by a practical boron–Wittig reaction. Org. Lett. 17, 1708–1711 (2015)
Balunas, M. J. et al. Coibacins A–D, antileishmanial marine cyanobacterial polyketides with intriguing biosynthetic origins. Org. Lett. 14, 3878–3881 (2012)
Tsukamoto, H., Uchiyama, T., Suzuki, T. & Kondo, Y. Palladium(0)-catalyzed direct cross-coupling reaction of allylic alcohols with aryl- and alkenylboronic acids. Org. Biomol. Chem. 6, 3005–3013 (2008)
Koh, M. J. et al. High-value alcohols and higher-oxidation-state compounds by catalytic Z-selective cross-metathesis. Nature 517, 181–186 (2015)
Xu, C., Shen, X. & Hoveyda, A. H. In situ methylene capping: A general strategy for efficient stereoretentive catalytic olefin metathesis. The concept, methodological implications, and applications to synthesis of biologically active compounds. J. Am. Chem. Soc. 139, 10919–10928 (2017)
Kolská, K., Ghavre, M., Pour, M., Hybelbauerová, S. & Kotora, M. Total synthesis of coibacin D by using enantioselective allylation and metathesis reactions. Asian J. Org. Chem. 5, 646–651 (2016)
Nunnery, J. K. et al. Biosynthetically intriguing chlorinated lipophilic metabolites from geographically distant tropical marine cyanobacteria. J. Org. Chem. 77, 4198–4208 (2012)
Romo, D. et al. Total synthesis and immunosuppressive activity of (–)-pateamine A and related compounds: implementation of a β-lactam-based macrocyclization. J. Am. Chem. Soc. 120, 12237–12254 (1998)
Northcote, P. T., Blunt, J. W. & Munro, M. H. G. Pateamine: a potent cytotoxin from the New Zealand marine sponge, Mycale sp. Tetrahedr. Lett. 32, 6411–6414 (1991)
Ondi, L., Nagy, G. M., Czirok, J. B., Bucsai, A. & Frater, G. E. From box to bench: Air-stable molybdenum catalyst tablets for everyday use in olefin metathesis. Org. Process Res. Dev. 20, 1709–1716 (2016)
Acknowledgements
This research was supported by the United States National Institutes of Health, Institute of General Medical Sciences (GM-59426 and, in part, CHE-1362763). M.J.K. and T.J.M. are grateful for support in the form of a Bristol Myers-Squibb Fellowship in Organic Chemistry and a John LaMattina Graduate Fellowship, respectively.
Author information
Authors and Affiliations
Contributions
T.T.N. and M.J.K. were involved in the discovery, design and development of the cross-metathesis strategies and their applications. T.J.M. carried out the initial exploratory studies with 1,1-disubstituted alkenes. A.H.H. designed and directed the investigations. A.H.H. and R.R.S. conceived the studies that led to the development of molybdenum complexes used in this study. A.H.H. wrote the manuscript with revisions provided by T.T.N., M.J.K. and T.J.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Non-productive olefin metathesis pathways.
Cross-metathesis between v and 1a via symmetrical metallacyclobutane iv′ (right, in black) is more likely than one involving complex iv″ as an intermediate (left, in red). This is as a result of greater steric pressure between the Cα substituent and the sizeable aryloxide ligand27. Cycloreversion of iv′ would then regenerate v and afford 1a (a non-productive process).
Extended Data Figure 2 Distinctive pathways for cross-metathesis of 22 and vinyl–B(pin) with Mo-1 and Mo-2.
a, Cross-metathesis between 25 and vinyl–B(pin) in the presence of Mo-1 and Mo-2 results in different product distribution and stereoselectivity profiles. b, The reactions proceed via mcbIMe because of severe steric repulsion between the larger Cβ aryl group in mcbIIMe and the Me units of the aryloxide ligand in Mo-3. By-product 33 may react with vinyl–B(pin) to furnish Z-32. c, There is less steric pressure at Cβ in mcbIt- Bu and mcbIIt- Bu; consequently, steric repulsion between the Cα metallacyclobutane substituent and an ortho fluorine substituent of the arylimido becomes more of a factor. Therefore, cross-metathesis probably proceeds via mcbIIt- Bu to afford the corresponding alkenyl–B(pin) compound (E-32). The resulting reaction of xiv with vinyl–B(pin) probably affords 34, which may then react with vinyl–B(pin) to furnish E-3n.
Supplementary information
Supplementary Information
This file contains all experimental and analytical data – see contents page for details. (PDF 13050 kb)
Rights and permissions
About this article
Cite this article
Nguyen, T., Koh, M., Mann, T. et al. Synthesis of E- and Z-trisubstituted alkenes by catalytic cross-metathesis. Nature 552, 347–354 (2017). https://doi.org/10.1038/nature25002
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature25002
This article is cited by
-
Photocatalytic Z/E isomerization unlocking the stereodivergent construction of axially chiral alkene frameworks
Nature Communications (2024)
-
Trisubstituted alkenes featuring aryl groups: stereoselective synthetic strategies and applications
Science China Chemistry (2023)
-
E- and Z-trisubstituted macrocyclic alkenes for natural product synthesis and skeletal editing
Nature Chemistry (2022)
-
Stereodefined alkenes with a fluoro-chloro terminus as a uniquely enabling compound class
Nature Chemistry (2022)
-
Nickel catalyzed multicomponent stereodivergent synthesis of olefins enabled by electrochemistry, photocatalysis and photo-electrochemistry
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.