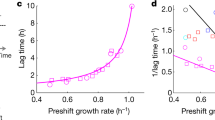
Abstract
A grand challenge of systems biology is to predict the kinetic responses of living systems to perturbations starting from the underlying molecular interactions. Changes in the nutrient environment have long been used to study regulation and adaptation phenomena in microorganisms1,2,3 and they remain a topic of active investigation4,5,6,7,8,9,10,11. Although much is known about the molecular interactions that govern the regulation of key metabolic processes in response to applied perturbations12,13,14,15,16,17, they are insufficiently quantified for predictive bottom-up modelling. Here we develop a top-down approach, expanding the recently established coarse-grained proteome allocation models15,18,19,20 from steady-state growth into the kinetic regime. Using only qualitative knowledge of the underlying regulatory processes and imposing the condition of flux balance, we derive a quantitative model of bacterial growth transitions that is independent of inaccessible kinetic parameters. The resulting flux-controlled regulation model accurately predicts the time course of gene expression and biomass accumulation in response to carbon upshifts and downshifts (for example, diauxic shifts) without adjustable parameters. As predicted by the model and validated by quantitative proteomics, cells exhibit suboptimal recovery kinetics in response to nutrient shifts owing to a rigid strategy of protein synthesis allocation, which is not directed towards alleviating specific metabolic bottlenecks. Our approach does not rely on kinetic parameters, and therefore points to a theoretical framework for describing a broad range of such kinetic processes without detailed knowledge of the underlying biochemical reactions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Monod, J. The phenomenon of enzymatic adaptation and its bearings on problems of genetics and cellular differentiation. Growth 11, 223–289 (1947)
Kjeldgaard, N. O., Maaløe, O. & Schaechter, M. The transition between different physiological states during balanced growth of Salmonella typhimurium. J. Gen. Microbiol. 19, 607–616 (1958)
Dennis, P. P. & Bremer, H. Regulation of ribonucleic acid synthesis in Escherichia coli: An analysis of a shift-up. J. Mol. Biol. 75, 145–159 (1973)
Thattai, M. & Shraiman, B. I. Metabolic switching in the sugar phosphotransferase system of Escherichia coli. Biophys. J. 85, 744–754 (2003)
Kussell, E. & Leibler, S. Phenotypic diversity, population growth, and information in fluctuating environments. Science 309, 2075–2078 (2005)
Acar, M., Mettetal, J. T. & van Oudenaarden, A. Stochastic switching as a survival strategy in fluctuating environments. Nat. Genet. 40, 471–475 (2008)
Boulineau, S. et al. Single-cell dynamics reveals sustained growth during diauxic shifts. PLoS One 8, e61686 (2013)
Kotte, O., Volkmer, B., Radzikowski, J. L. & Heinemann, M. Phenotypic bistability in Escherichia coli’s central carbon metabolism. Mol. Syst. Biol. 10, 736 (2014)
van Heerden, J. H. et al. Lost in transition: start-up of glycolysis yields subpopulations of nongrowing cells. Science 343, 1245114 (2014)
Venturelli, O. S., Zuleta, I., Murray, R. M. & El-Samad, H. Population diversification in a yeast metabolic program promotes anticipation of environmental shifts. PLoS Biol. 13, e1002042 (2015)
Link, H., Fuhrer, T., Gerosa, L., Zamboni, N. & Sauer, U. Real-time metabolome profiling of the metabolic switch between starvation and growth. Nat. Methods 12, 1091–1097 (2015)
Kolb, A., Busby, S., Buc, H., Garges, S. & Adhya, S. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62, 749–795 (1993)
Potrykus, K. & Cashel, M. (p)ppGpp: still magical? Annu. Rev. Microbiol. 62, 35–51 (2008)
Lemke, J. J. et al. Direct regulation of Escherichia coli ribosomal protein promoters by the transcription factors ppGpp and DksA. Proc. Natl Acad. Sci. USA 108, 5712–5717 (2011)
You, C. et al. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 500, 301–306 (2013)
Scott, M., Klumpp, S., Mateescu, E. M. & Hwa, T. Emergence of robust growth laws from optimal regulation of ribosome synthesis. Mol. Syst. Biol. 10, 747 (2014)
Hauryliuk, V., Atkinson, G. C., Murakami, K. S., Tenson, T. & Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 13, 298–309 (2015)
Scott, M., Gunderson, C. W., Mateescu, E. M., Zhang, Z. & Hwa, T. Interdependence of cell growth and gene expression: origins and consequences. Science 330, 1099–1102 (2010)
Hui, S. et al. Quantitative proteomic analysis reveals a simple strategy of global resource allocation in bacteria. Mol. Syst. Biol. 11, 784 (2015)
Basan, M. et al. Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature 528, 99–104 (2015)
Hermsen, R., Okano, H., You, C., Werner, N. & Hwa, T. A growth-rate composition formula for the growth of E. coli on co-utilized carbon substrates. Mol. Syst. Biol. 11, 801 (2015)
Chubukov, V., Gerosa, L., Kochanowski, K. & Sauer, U. Coordination of microbial metabolism. Nat. Rev. Microbiol. 12, 327–340 (2014)
Reitzer, L. Nitrogen assimilation and global regulation in Escherichia coli. Annu. Rev. Microbiol. 57, 155–176 (2003)
Dai, X. et al. Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat. Microbiol. 2, 16231 (2016)
Gausing, K. Ribosomes: Structure, Function and Genetics 693–718 (Univ. Park Press, 1980)
Postma, P. W., Lengeler, J. W. & Jacobson, G. R. Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57, 543–594 (1993)
Pavlov, M. Y. & Ehrenberg, M. Optimal control of gene expression for fast proteome adaptation to environmental change. Proc. Natl Acad. Sci. USA 110, 20527–20532 (2013)
Giordano, N., Mairet, F., Gouzé, J.-L., Geiselmann, J. & de Jong, H. Dynamical allocation of cellular resources as an optimal control problem: novel insights into microbial growth strategies. PLoS Comput. Biol. 12, e1004802 (2016)
Csonka, L. N., Ikeda, T. P., Fletcher, S. A. & Kustu, S. The accumulation of glutamate is necessary for optimal growth of Salmonella typhimurium in media of high osmolality but not induction of the proU operon. J. Bacteriol. 176, 6324–6333 (1994)
Soupene, E. et al. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J. Bacteriol. 185, 5611–5626 (2003)
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000)
Miller, J. H. Experiments in Molecular Genetics (Cold Spring Harbor Laboratory Press, 1972)
Benthin, S., Nielsen, J. & Villadsen, J. A simple and reliable method for the determination of cellular RNA content. Biotechnol. Tech. 5, 39–42 (1991)
Deutsch, E. W. et al. A guided tour of the Trans-Proteomic Pipeline. Proteomics 10, 1150–1159 (2010)
Craig, R. & Beavis, R. C. TANDEM: matching proteins with tandem mass spectra. Bioinformatics 20, 1466–1467 (2004)
Sperling, E., Bunner, A. E., Sykes, M. T. & Williamson, J. R. Quantitative analysis of isotope distributions in proteomic mass spectrometry using least-squares Fourier transform convolution. Anal. Chem. 80, 4906–4917 (2008)
Sykes, M. T., Sperling, E., Chen, S. S. & Williamson, J. R. Quantitation of the ribosomal protein autoregulatory network using mass spectrometry. Anal. Chem. 82, 5038–5045 (2010)
Schaechter, M., Maaløe, O. & Kjeldgaard, N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J. Gen. Microbiol. 19, 592–606 (1958)
Basan, M. et al. Inflating bacterial cells by increased protein synthesis. Mol. Syst. Biol. 11, 836 (2015)
Tong, S., Porco, A., Isturiz, T. & Conway, T. Cloning and molecular genetic characterization of the Escherichia coli gntR, gntK, and gntU genes of GntI, the main system for gluconate metabolism. J. Bacteriol. 178, 3260–3269 (1996)
Voegele, R. T., Sweet, G. D. & Boos, W. Glycerol kinase of Escherichia coli is activated by interaction with the glycerol facilitator. J. Bacteriol. 175, 1087–1094 (1993)
Dennis, P. P. & Bremer, H. Differential rate of ribosomal protein synthesis in Escherichia coli B/r. J. Mol. Biol. 84, 407–422 (1974)
Acknowledgements
We are grateful to E. Mateescu for initiating this work and to J. Hasty and R. Young for discussions. This work is supported by the National Institutes of Health (NIH; grant 1R01GM109069) and by the Simons Foundation (grant 330378) through T.H., by the NIH (grant 1R01GM118850) through J.R.W., and by the German Research Foundation via the Excellence Cluster ‘Nanosystems Initiative Munich’ and the priority program SPP1617 (grant GE1098/6-2) through U.G.
Author information
Authors and Affiliations
Contributions
D.W.E., S.J.S., U.G. and T.H. designed this study. Growth shifts were performed by D.W.E. and S.J.S. and analysed by S.J.S., D.W.E. and T.H. Quantitative mass spectrometry analysis was performed by V.P. and analysed by S.J.S., V.P. and J.R.W. D.W.E., S.J.S., U.G. and T.H. developed the model and all authors participated in writing the paper and the Supplementary Information.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Growth laws.
a, Dry mass per optical density OD600 nm is independent of the growth rate in the investigated conditions with an average of 509 μg dry weight per ml × OD600 nm value (black line). b, Protein mass per optical density OD600 nm shows a slight dependence on growth rate (black line: guide to the eye), in accordance with the increase of RNA and DNA in the cell at higher growth rates38. Because the change is small in the range of growth rates of the up- and downshifts presented, we take the conversion from dry mass to total protein to be constant throughout the shifts presented. Dry mass and total protein data taken from ref. 39. c, Ribosomal proteome fraction of the cell  at steady-state growth at rate λ*, using RNA or protein as a reporter. The data are fitted with the linear relation
at steady-state growth at rate λ*, using RNA or protein as a reporter. The data are fitted with the linear relation with φRb,0 = (0.049 ± 0.02) and γ = (11.02 ± 0.44) h−1. d, Catabolic proteins (
with φRb,0 = (0.049 ± 0.02) and γ = (11.02 ± 0.44) h−1. d, Catabolic proteins ( , left axis) were measured with LacZ (induced with 1 mM IPTG) as a reporter and measuring Miller units (MU) (right axis; see ref. 32 for MU). The linear relation was fitted with
, left axis) were measured with LacZ (induced with 1 mM IPTG) as a reporter and measuring Miller units (MU) (right axis; see ref. 32 for MU). The linear relation was fitted with  with
with  and λC = (1.17 ± 0.05) h−1. e, The translational activity σ* is calculated from
and λC = (1.17 ± 0.05) h−1. e, The translational activity σ* is calculated from  (c) as
(c) as  f, g, Regulatory functions
f, g, Regulatory functions  and
and  , functions of the translational activity σ*, are calculated by substituting λ* by σ* from the fits in c and d (see Supplementary equations (28) and (29)). h, i, Tables show all data plotted in c and d. Data in h were measured by growth on different carbon substrates, data in i were measured by growth of strains with titratable transporter expression, see list of strains in j.
, functions of the translational activity σ*, are calculated by substituting λ* by σ* from the fits in c and d (see Supplementary equations (28) and (29)). h, i, Tables show all data plotted in c and d. Data in h were measured by growth on different carbon substrates, data in i were measured by growth of strains with titratable transporter expression, see list of strains in j.
Extended Data Figure 2 Long-term dynamics and repeatability.
a, Serial dilution experiment of the upshift of Fig. 1 (succinate, add gluconate). The top graph shows optical density OD600 nm as a measure of biomass M(t), the middle graph shows biomass flux J(t), and the bottom graph shows the growth rate λ(t) = J(t)/M(t). Shortly after the upshift, the cell culture was diluted 4- and 16-fold in fresh medium, grown in parallel to the original culture, and the optical density was recorded for the diluted cultures once they reached an optical density of around 0.05. As the growth rate is independent of the cell density, data from the original and the diluted cultures collapse, showing that for longer times the growth rate reaches its final level (dashed line). b, Repeatability. Biomass (top), flux (middle) and growth rate (bottom) for (left to right) the upshift from Fig. 1a–e and the downshift shown in Fig. 1f–j. Theory (black line) and data (red diamonds) are identical to Fig. 1. Four independent repeats (purple, orange, green and blue) are plotted on top of the data shown in Fig. 1.
Extended Data Figure 3 Upshifts from succinate and pyruvate.
a–h, NCM3722 grown exponentially on 20 mM succinate (succ.) or 20 mM pyruvate (pyr.) as the sole carbon substrate. At t = 0 a second, subsequently co-utilized carbon substrate was added: 0.2% arabinose (a, e); 0.2% xylose (b, f); 0.2% glycerol (c, g); 0.2% glucose (d); 20 mM gluconate (h). The left panels show optical density OD600 nm (a measure of biomass M(t), red circles), the middle panels show the derivative of OD600 nm (a measure for biomass flux J(t), red squares), and the right panels show growth rate (λ(t) = J(t)/M(t), red diamonds). Theory lines for M(t), J(t) and λ(t) were calculated using Supplementary equations (76), (70) and (77), using the initial condition Supplementary equation (52) for upshift without pre-expression. Initial and final growth rates were measured during steady-state growth on the respective carbon substrates.
Extended Data Figure 4 Downshift with co-utilized carbon substrates.
a–d, NCM3722 grown exponentially on 20 mM succinate (succ.) (a, b) or 20 mM pyruvate (pyr.) (c, d), combined with either 0.56 mM gluconate (gluc.) (a, c) or 1.11 mM glycerol (glyc.) (b, d). At around t = 0, gluconate or glycerol were depleted. The left panels show optical density OD600 nm (a measure of biomass M(t), red circles), middle panels show the derivative of OD600 nm (a measure for biomass flux J(t), red squares), and right panels show the growth rate (λ(t) = J(t)/M(t), red diamonds). Theory lines for M(t), J(t) and λ(t) were calculated solving the differential equation for the translational activity σ(t) (Supplementary equation (22)), and those defining the internal fluxes and protein content (Supplementary equations (19)–(21) and (22)–(24)) numerically. The uptake of the depleting substrate (glycerol or gluconate) was calculated from Supplementary equation (20), using the Michaelis constants Km = 212 μM (gluconate40) and Km = 5.6 μM (glycerol41). Since gluconate transport has a low affinity, it depletes slowly (see a and c), which is accurately described by our modelling of catabolism. Initial and final growth rates were measured during steady-state growth on the respective carbon substrates.
Extended Data Figure 5 Performance of models of growth kinetics without gene regulation.
a–c, This figure shows the result of three nutrient shifts discussed in the main text: Succinate–gluconate upshift from Fig. 1b (a); Succinate–glucose downshift from Fig. 1g (b); Glucose–lactose diauxie shift from Fig. 3b (c). Experimental data are shown as red symbols. Among existing models of growth transition, the one proposed by Dennis and Bremer allows for predicting growth transitions of upshifts42. This model assumes that the flux jumps instantaneously to the final state without a regulatory scheme (see Supplementary Note 1 for a review). Predictions of this model, referred to here as the ‘instantaneous model’, are shown as the green dashed lines in all panels. Although the instantaneous model works well for upshifts (panel a), for which it was developed42, it fails to describe downshifts (panels b and c), because it cannot describe a flux decrease. The instantaneous model cannot be fixed by matching the flux after glucose depletion (blue dotted lines in panels b and c), as it underestimates the recovery rate owing to a lacking regulatory scheme (Supplementary Note 1). By contrast, the FCR model introduced in this work, based on an active reallocation of protein synthesis during growth transitions, predicts the correct recovery rates (black solid lines) consistently for both up- and downshifts.
Extended Data Figure 6 Upshift from mannose.
a–c, NCM3722 grown exponentially on 0.1% mannose as the sole carbon substrate. At t = 0 (dashed line) oxaloacetic acid (OAA), subsequently co-utilized, is added. Optical density OD600 nm (a measure of biomass M(t), red circles) (a), the derivative of OD600 nm (a measure of biomass flux J(t), red squares) (b), growth rate (λ(t) = J(t)/M(t), red diamonds) (c). d, Expression of the catabolic proteins reporter LacZ is transiently repressed after the shift (vertical dashed line). Dotted black lines show solutions of the theory using the initial condition for no pre-expression (Supplementary equation (52)), which does not coincide with the experimental data (symbols). Using the initial condition for pre-expression (Supplementary equation (54)) and fitting the pre-expression level, the theory (black lines) describes the data very well, including the transient repression of LacZ in d. LacZ activity units U are defined as MU × OD600 nm.
Extended Data Figure 7 Effect of pre-expression on upshift kinetics.
a, E. coli NQ530 grown exponentially in mannose minimal medium with different levels of succinate transporter (DctA) pre-expressed via the titratable Pu promoter (regulator: XylR, inducer: 3MBA). At time t = 0, medium was supplemented with 20 mM succinate and DctA expression is set to a common level for all upshifts. b, Biomass M(t) (OD600 nm), biomass flux J(t) (derivative of OD600 nm) and growth rate λ(t) for different levels of pre-expression (3MBA concentration indicated in panels). DctA pre-expression positively affects post-shift growth. The biomass flux J(t) (middle) shows a saltatory increase followed by exponential growth at final rate λf. For cultures with a high level of pre-expression, the instantaneous growth rate λ(t) (right) transiently overshot before relaxing to the final value λf. The increase is transient because the DctA level in the synthetic construct eventually decreases below the pre-expression level. The kinetic theory quantitatively captures the upshift kinetics for all pre-expression levels, when using the initial condition σ(0) as the single fit parameter (solid lines). (c) NQ 1324 (∆dctA) shows a small increase in growth rate upon upshift, despite the succinate transporter DctA being knocked out. Final growth rate is not significantly increased over steady-state growth on mannose alone. Steady-state growth rate on succinate alone was barely detectable for NQ1324 ( ). e, Validation of theoretical prediction. The magnitude of saltatory increase in growth rate Δλ (indicated in the right panels of b, c) depends linearly on the pre-expression levels. The saltatory increase of the dctA knockout shown in c is indicated by a triangle.
). e, Validation of theoretical prediction. The magnitude of saltatory increase in growth rate Δλ (indicated in the right panels of b, c) depends linearly on the pre-expression levels. The saltatory increase of the dctA knockout shown in c is indicated by a triangle.
Extended Data Figure 8 Proteome remodelling.
a, b, Regulatory functions χ and protein fractions φ of catabolic proteins (relative to maximal expression), ribosomes (absorbance in mg RNA per mg protein) as well as growth rate λ(t) and translational activity σ(t) during growth shifts for the upshift and downshift of Fig. 1. Soon after upshift the regulatory functions of catabolic proteins χCat(t) and ribosomes χRb(t), driven by the translational activity σ(t) (green, blue and red dashed lines), have relaxed to the final state (a). A new proteome is thus synthesized at the final ratio, leading to a slow convergence to the final state by growth-mediated dilution. The shortfalls of carbon influx after glucose depletion lead to an upregulation of χCat(t) and a downregulation of χRb(t), driven by the translational activity σ(t) (green, blue and red dashed lines) (b). c, The regulatory functions χCat(t) and χRb(t), set by the translational activity σ(t), plotted versus growth rate λ(t). Thin black lines show the steady-state growth laws (Extended Data Fig. 1c, d and Supplementary Note 2). Despite the regulatory functions being derived from the growth laws (black lines), they diverge considerably during growth transitions, as they are controlled by the translational activity σ(t), and not the growth rate λ(t). d, Assuming co-regulation of the proteome sectors C↑ and C↓ with catabolic and ribosomal proteins (equations (6a) and (6b)), the dynamics of the proteome sectors can be described (equations (7a) and (7b); see Supplementary Note 5 for details). e, f, Graphical synopsis of the proteome remodelling of a and b, as predicted by equations (6a), (6b), (7a) and (7b). Red boundary and arrows show dynamics. In upshifts regulatory functions χ rapidly relax to their final states; in downshifts regulatory functions initially overshoot and relax slowly to their final states (see Supplementary Note 6 for extended discussion).
Extended Data Figure 9 Biphasic relaxation kinetics.
The figure shows the relaxation kinetics for growth shifts, as obtained from the FCR model. a–c, An upshift from slow growth λi = 0.3 h−1 (orange) and a downshift from fast pre-shift growth λi = 0.95 h−1 (green) is seen, both with the same growth rate directly after the shift, λ(0) = 0.3 h−1, and the same fast final growth rate λf = 0.95 h−1. d–f, An upshift from slow growth λi = 0.2 h−1 (orange) and a downshift from fast pre-shift growth λi = 0.95 h−1 (green) is seen, with λ(0) = 0.2 h−1, and the same slow growth rate λf = 0.95 h−1. a, d, The trajectories of the recovery in the space of growth rate (left y-axis), which is proportional to the catabolic protein fraction (right y-axis), and ribosome fraction φRb(t) (bottom x-axis). The initial condition φRb(0) depends on the pre-shift growth rate λi (top x-axis). The trajectories start at the points (φRb(0), λ(0)), indicated by coloured squares, and end at the final state (φRb,f, λf), marked with a black cross. As detailed in Supplementary Note 6, the kinetics are biphasic and can be understood using a simple geometric construction, which yields the dotted and dashed lines (see Supplementary Note 6.2). First, a relaxation associated with a timescale μf−1 (Supplementary equation (116)), along the grey dashed lines, during which protein synthesis contains more catabolic proteins and fewer ribosomes than the final composition of the proteome. The end of this kinetic phase is indicated by circles in all panels. Second, a slow motion along the diagonal dashed line on the timescale λf−1 (Supplementary equation (120)). Along this line, the translational activity, σ(t) = λ(t)/φRb(t), has relaxed to the final state (see b, e), where σ(t) has relaxed after the ‘circles’. As a result, the regulatory functions have relaxed to the final state too, and protein synthesis contains the same amount of catabolic proteins and ribosomes as in the final state. During this second phase, the proteome gradually adapts due to growth-mediated dilution of inherited proteins. c, f, The growth rate almost fully adapts during the first phase (from t = 0 to the circle) for the downshifts (green, cyan), but not for the upshifts (orange, purple). This difference is because of the high ribosome abundance in downshifts, which allows increased expression of catabolic proteins, and thus almost entirely avoids the slow phase, and is more prominent for shifts to fast final growth (a–c), than to slow final growth (d–f).
Extended Data Figure 10 Protein degradation.
a, Pulse-labelling allows the differentiation of pre-pulse and post-pulse protein mass. We added concentrated 15NH4Cl into the culture at the moment of gluconate upshift or a few minutes before glucose exhaustion during the downshift. Comparing to a third isotope species (spiked-in 15N-reference culture) allows tracking the levels of pre-shift proteins over time. b, A schematic showing the levels of total cellular protein (black) and cellular protein existing at pulse time (blue) as the culture is instantaneously upshifted. Stable protein levels are characterized by a zero-slope line (blue solid line), whereas degrading or exported cellular proteins exhibit a negative-slope line (blue dashed line, red arrow). c, The post-shift levels of 40 cellular proteins of highest mass fraction were quantified using the pulse-labelling approach. Light (L, 14N) over heavy (H, 15N) relative protein levels are plotted as a function of time. These proteins span diverse biological functions, cellular localization, size and structure. Together, they account for 35–40% of the total protein mass detected throughout the shift (estimated by summing their mass abundances listed in Supplementary Table 2 for each condition). With the exception of flagellin (fliC, red box), we did not observe decreasing protein levels for either the upshift or downshift series on the 2-h timescale for large-abundance proteins, which we could confidently quantify. Flagellin is exported to the cell periphery by a dedicated transport system, and probably shed into the medium during steady-state growth. As shed proteins are not collected at the same efficiency as proteins in cells, the decline of pre-labelled FliC serves as a positive control for the method.
Supplementary information
Supplementary Information
This file contains full descriptions for Supplementary Tables 1–4, Supplementary Notes and Supplementary references – see contents page for details.
Supplementary Table 1
This table contains relative expression data, see Supplementary Information document for full description.
Supplementary Table 2
This table contains absolute expression data, see Supplementary Information document for full description.
Supplementary Table 3
This table shows proteome sectors, see Supplementary Information document for full description.
Supplementary Table 4
This table shows protein groups, see Supplementary Information document for full description.
Rights and permissions
About this article
Cite this article
Erickson, D., Schink, S., Patsalo, V. et al. A global resource allocation strategy governs growth transition kinetics of Escherichia coli. Nature 551, 119–123 (2017). https://doi.org/10.1038/nature24299
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature24299
This article is cited by
-
A coarse-grained bacterial cell model for resource-aware analysis and design of synthetic gene circuits
Nature Communications (2024)
-
Shaping bacterial gene expression by physiological and proteome allocation constraints
Nature Reviews Microbiology (2023)
-
Functional decomposition of metabolism allows a system-level quantification of fluxes and protein allocation towards specific metabolic functions
Nature Communications (2023)
-
Unbalanced response to growth variations reshapes the cell fate decision landscape
Nature Chemical Biology (2023)
-
Dynamic proteome trade-offs regulate bacterial cell size and growth in fluctuating nutrient environments
Communications Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.