Abstract
Locomotion is a universal behaviour that provides animals with the ability to move between places. Classical experiments have used electrical microstimulation to identify brain regions that promote locomotion1,2,3,4,5, but the identity of neurons that act as key intermediaries between higher motor planning centres and executive circuits in the spinal cord has remained controversial6,7,8,9,10,11,12,13,14. Here we show that the mouse caudal brainstem encompasses functionally heterogeneous neuronal subpopulations that have differential effects on locomotion. These subpopulations are distinguishable by location, neurotransmitter identity and connectivity. Notably, glutamatergic neurons within the lateral paragigantocellular nucleus (LPGi), a small subregion in the caudal brainstem, are essential to support high-speed locomotion, and can positively tune locomotor speed through inputs from glutamatergic neurons of the upstream midbrain locomotor region. By contrast, glycinergic inhibitory neurons can induce different forms of behavioural arrest mapping onto distinct caudal brainstem regions. Anatomically, descending pathways of glutamatergic and glycinergic LPGi subpopulations communicate with distinct effector circuits in the spinal cord. Our results reveal that behaviourally opposing locomotor functions in the caudal brainstem were historically masked by the unexposed diversity of intermingled neuronal subpopulations. We demonstrate how specific brainstem neuron populations represent essential substrates to implement key parameters in the execution of motor programs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shik, M. L. & Orlovsky, G. N. Neurophysiology of locomotor automatism. Physiol. Rev. 56, 465–501 (1976)
Skinner, R. D. & Garcia-Rill, E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 323, 385–389 (1984)
Mori, S., Matsuyama, K., Mori, F. & Nakajima, K. Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv. Neurol. 87, 25–40 (2001)
Noga, B. R., Kriellaars, D. J., Brownstone, R. M. & Jordan, L. M. Mechanism for activation of locomotor centers in the spinal cord by stimulation of the mesencephalic locomotor region. J. Neurophysiol. 90, 1464–1478 (2003)
Jordan, L. M., Liu, J., Hedlund, P. B., Akay, T. & Pearson, K. G. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 57, 183–191 (2008)
Drew, T. & Rossignol, S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J. Neurophysiol. 64, 767–781 (1990)
Kinjo, N. et al. Medioventral medulla-induced locomotion. Brain Res. Bull. 24, 509–516 (1990)
Noga, B. R., Kettler, J. & Jordan, L. M. Locomotion produced in mesencephalic cats by injections of putative transmitter substances and antagonists into the medial reticular formation and the pontomedullary locomotor strip. J. Neurosci. 8, 2074–2086 (1988)
Garcia-Rill, E. & Skinner, R. D. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 411, 1–12 (1987)
Ross, G. S. & Sinnamon, H. M. Forelimb and hindlimb stepping by the anesthetized rat elicited by electrical stimulation of the pons and medulla. Physiol. Behav. 33, 201–208 (1984)
Hajnik, T., Lai, Y. Y. & Siegel, J. M. Atonia-related regions in the rodent pons and medulla. J. Neurophysiol. 84, 1942–1948 (2000)
Mori, S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog. Neurobiol. 28, 161–195 (1987)
Takakusaki, K., Chiba, R., Nozu, T. & Okumura, T. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J. Neural Transm. (Vienna) 123, 695–729 (2016)
Orlovsky, G. N ., Deliagina, T. G. & Grillner, S. Neuronal Control of Locomotion: from Mollusc to Man Ch. 12, 205–214 (Oxford Univ. Press, 1999)
Wickersham, I. R., Finke, S., Conzelmann, K. K. & Callaway, E. M. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 4, 47–49 (2007)
Franklin, K. B. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates 3rd edn (Elsevier, 2007)
Liang, H., Paxinos, G. & Watson, C. Projections from the brain to the spinal cord in the mouse. Brain Struct. Funct. 215, 159–186 (2011)
Esposito, M. S., Capelli, P. & Arber, S. Brainstem nucleus MdV mediates skilled forelimb motor tasks. Nature 508, 351–356 (2014)
Drew, T. Functional organization within the medullary reticular formation of the intact unanesthetized cat. III. Microstimulation during locomotion. J. Neurophysiol. 66, 919–938 (1991)
Drew, T. & Rossignol, S. Functional organization within the medullary reticular formation of intact unanesthetized cat. II. Electromyographic activity evoked by microstimulation. J. Neurophysiol. 64, 782–795 (1990)
Bouvier, J. et al. Descending command neurons in the brainstem that halt locomotion. Cell 163, 1191–1203 (2015)
Schoenenberger, P., Gerosa, D. & Oertner, T. G. Temporal control of immediate early gene induction by light. PLoS One 4, e8185 (2009)
Grillner, S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52, 751–766 (2006)
Kiehn, O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 17, 224–238 (2016)
Goulding, M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507–518 (2009)
Weber, F. et al. Control of REM sleep by ventral medulla GABAergic neurons. Nature 526, 435–438 (2015)
Lee, A. M. et al. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83, 455–466 (2014)
Roseberry, T. K. et al. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell 164, 526–537 (2016)
Kropff, E., Carmichael, J. E., Moser, M. B. & Moser, E. I. Speed cells in the medial entorhinal cortex. Nature 523, 419–424 (2015)
Fuhrmann, F. et al. Locomotion, theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron 86, 1253–1264 (2015)
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011)
Foster, E. et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 85, 1289–1304 (2015)
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011)
Zeilhofer, H. U. et al. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J. Comp. Neurol. 482, 123–141 (2005)
Osakada, F. & Callaway, E. M. Design and generation of recombinant rabies virus vectors. Nat. Protocols 8, 1583–1601 (2013)
Stepien, A. E., Tripodi, M. & Arber, S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron 68, 456–472 (2010)
Wickersham, I. R. et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (2007)
Pivetta, C., Esposito, M. S., Sigrist, M. & Arber, S. Motor-circuit communication matrix from spinal cord to brainstem neurons revealed by developmental origin. Cell 156, 537–548 (2014)
Basaldella, E., Takeoka, A., Sigrist, M. & Arber, S. Multisensory signaling shapes vestibulo-motor circuit specificity. Cell 163, 301–312 (2015)
Takeoka, A., Vollenweider, I., Courtine, G. & Arber, S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159, 1626–1639 (2014)
Satoh, D., Pudenz, C. & Arber, S. Context-dependent gait choice elicited by EphA4 mutation in Lbx1 spinal interneurons. Neuron 89, 1046–1058 (2016)
Fenno, L. E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014)
Lin, J. Y., Knutsen, P. M., Muller, A., Kleinfeld, D. & Tsien, R. Y. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508 (2013)
Tervo, D. G. et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382 (2016)
Dietz, C. & Berthold, M. R. KNIME for Open-Source Bioimage Analysis: A Tutorial. Adv. Anat. Embryol. Cell Biol. 219, 179–197 (2016)
Acknowledgements
We are grateful to M. Sigrist, M. Mielich, M. Pinto, J. Alonso, C. Hartmann and M. Theodore for experimental help, P. Tovote for advice with optogenetic technologies, S. Valencia Garcia and E. Arteaga Bracho for advice on EMG recordings, E. Jankowska for advice on cat brainstem nomenclature, A. Karpova for sharing the rAAV2-retro capsid plasmid before publication, R. Thierry and J. Eglinger from the FMI imaging facility and N. Ehrenfeuchter from the Biozentrum imaging facility for help and advice with image acquisition and analysis, P. Argast and P. Buchmann from the FMI mechanical workshop for building devices for behavioural experiments, M. Stadler and D. Gaidatzis for help with statistical analysis, and P. Caroni for discussions and comments on the manuscript. All authors were supported by an ERC Advanced Grant, the Swiss National Science Foundation, Kanton Basel-Stadt and Novartis Research Foundation, and the Louis Jeantet Prize for Medicine. M.S.E. was also supported by an HFSP long-term postdoctoral fellowship, Synapsis Foundation Grant and NARSAD Young Investigator Grant by the Brain and Behavior Foundation.
Author information
Authors and Affiliations
Contributions
All authors were involved in the design of experiments. P.C. together with C.P. carried out most experiments, acquired and analysed data. M.S.E. carried out experiments related to MLR and MLR–Mc interactions, was involved in EMG and kinematic experiments, acquired and analysed data. S.A. initiated the project, designed experiments, analysed data and wrote the manuscript. All authors discussed the experiments and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Subdivision of lumbar projection neurons in caudal medulla.
Views of three-dimensional reconstruction of rabies-FP-marked cell body positions of neurons projecting to the lumbar spinal cord, depicted with a colour code for different regions as described in Fig. 1b, c.
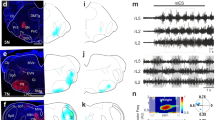
Extended Data Figure 2 Injection site location for unconditional optogenetic stimulation of brainstem neurons.
a–e, Analysis of injection and corresponding optic fibre positions targeting LPGi (a), GiA (b), GiV (c), Gi (d) and MLR (e) in wild-type mice is shown on brain atlas sections aligning with sites identified in corresponding experiments. Crosses depict centre of injections and rectangles show optic fibre tip positions, with each colour representing a different mouse included in the analysis shown in Fig. 1e. Bottom row shows representative pictures of YFP fluorescence after injections of AAV-flex-ReaChR-YFP. The mouse brain atlas images in this figure have been reproduced with permission from Elsevier.
Extended Data Figure 3 Injection site location for optogenetic stimulation of glutamatergic Mc and Gi neurons.
a–d, Analysis of injection and corresponding optic fibre positions targeting LPGi (a), GiA (b), GiV (c) and Gi (d) in vGlut2cre mice is shown on brain atlas sections aligning with sites identified in corresponding experiments. Crosses depict centre of injections and rectangles show optic fibre tip positions, with each colour representing a different mouse included in the analysis shown in Fig. 2 and Extended Data Fig. 5. Top row shows representative pictures of YFP fluorescence after injections of AAV-flex-ReaChR-YFP, and neighbouring motor nuclei (red). The mouse brain atlas images in this figure have been reproduced with permission from Elsevier.
Extended Data Figure 4 Injection site location for optogenetic stimulation of inhibitory Mc and Gi neurons.
a–d, Analysis of injection and corresponding optic fibre positions targeting LPGi (a), GiA (b), GiV (c) and Gi (d) in mice expressing Cre recombinase from loci of inhibitory neurotransmitters is shown on brain atlas sections aligning with sites identified in corresponding experiments. Crosses, circles and stars depict centre of injections in different genotypes and rectangles show optic fibre tip positions (legend bottom left), with each colour representing a different mouse included in the analysis shown in Fig. 2 and Extended Data Fig. 6. The mouse brain atlas images in this figure have been reproduced with permission from Elsevier.
Extended Data Figure 5 Stimulation of glutamatergic LPGi neurons elicits locomotion.
a, Top, stereotaxic injection of AAV-flex-ReaChR into LPGi, GiA, GiV or Gi of vGlut2cre mice. Bottom, blue laser stimulation of infected neurons induces Fos expression as proxy for neuronal activation in LPGi or GiA neurons. b, Centre of body mass trajectories of single trials in open field arena during two 1-s time windows: stationary phase (before, orange) and laser application phase (laser ON, cyan) centred to the starting position (top; 0.1 and 2 mW laser power). c, d, Average speed of single trials with 2 mW laser intensity (c), maximal speed during laser ON (d; left) and latency to initiate locomotion after laser onset (d; right) at different laser intensities for one representative example mouse. *P < 0.05, ***P < 0.001, one-way ANOVA/Bonferroni. e, Example of instantaneous speed of single mice (grey lines) and average of 4 mice (magenta) during 1-s optogenetic stimulation of LPGi-vGlut2 neurons (blue box), as well as 1 s before and after laser stimulation. f, A group of mice was injected with AAV-flex-GFP in LPGi as a control experiment (analysis as shown in Fig. 2h–j). g, Histogram of duration (left) and latency to stop (right; 0 s denotes laser offset) of individual locomotor bouts pooled from 3 mice induced by optogenetic stimulation of LPGi-vGlut2 neurons. h, Triceps EMG analysis during natural running wheel locomotion of LPGi-vGlut2 mice (compare to Fig. 2e). i–k, Direction of natural (NL) or laser-induced locomotion analysis in the open field arena (see Methods for analysis details). i, Experimental scheme to describe applied analysis (i, left) and frequency plot of angles (shown angle range covers 99% of all analysed trials) for a representative animal (i, middle) and over 3 animals (i, right). j, Percentage of angles in the range between −6 and +6°. k, Ratio of shortest to real distance travelled calculated as indicated in i. l, m, Kinematic analysis of natural and laser-induced locomotion of vGlut2cre mouse injected with AAV-flex-ReaChR-YFP in LPGi at a 40–50 cm s−1 speed, including principle component analysis (l; grey denotes swing phase; black denotes stance phase; n.s., not significant, non parametric t-test) and hindlimb (HL) and forelimb (FL) oscillations (m; grey box denotes swing phase of left (L) hindlimb). n–p, Analysis of running wheel (RW) locomotion in vGlut2cre mice with ReaChR expression targeted to LPGi neurons. n, o, In stationary mice, the application of laser light triggers locomotion during 1-s (n) or 5-s (o) laser stimulation (single trials are in grey, the average of analysed trials is in magenta). AU, arbitrary units. Plots at the bottom show the increasing running wheel speed for higher laser intensities of a single animal (left; **P < 0.01, ***P < 0.001, paired t-test) or group data (right; ***P < 0.001, two-way ANOVA/Bonferroni) upon 1-s stimulation (n), and maintained locomotion throughout 5 s of stimulation (o). p, For a LPGi-vGlut2-ReaChR mouse already running on the wheel, the application of a 5-mW laser can further increase speed. ***P < 0.001, paired t-test. q, Single trial average speed profiles to determine the effects of laser stimulation of vGlut2ON GiA (top), GiV (middle) or Gi (bottom) neurons on locomotor speed (1 s before, during and after laser). *P < 0.05, one-way ANOVA/Bonferroni). Data are mean ± s.e.m., for sample sizes see Supplementary Table 1.
Extended Data Figure 6 Stimulation of inhibitory Mc and Gi neurons.
a, b, Locomotor speed analysis of mice expressing ReaChR in LPGi-vGAT (a) or LPGi-GlyT2 (b) neurons in the open field arena. Left, trajectories of centre of body mass tracking for individual trials at 5 mW laser intensity 1 s before (orange), 1 s during (cyan), and 1 s after (magenta) laser application are shown aligned to a central point. Right, representative single trial speed analysis 1 s before and during laser application. ***P < 0.001, paired t-test. c, Top, example of single trials (grey lines) and average (dotted black line) of one mouse. Bottom, group data depicting averages of single mice (grey lines) and of 3 mice (magenta) during 1-s optogenetic stimulation of LPGi-GlyT2 neurons (blue box), as well as 1 s before and after laser stimulation. d, Average speed before (orange) and during (cyan) 1-s laser application at different laser intensities upon stimulation of LPGi-GlyT2 neurons for different mice analysed. *P < 0.05, **P < 0.01, unpaired t-test comparing data before and during laser application at each intensity. e, Locomotor speed analysis of mice expressing ReaChR in LPGi-Gad65 neurons in the open field arena. Left, trajectories of centre of body mass tracking for individual trials at 20 mW laser intensity 1 s before (orange) and 1 s during (cyan) laser application are shown aligned to a central point. Right, speed analysis 1 s before and during laser application. Not significant, one-way ANOVA/Bonferroni. f, g, Analysis of running wheel locomotion experiment in GlyT2cre (f) or Gad65cre (g) mice with ReaChR expression targeted to LPGi neurons. *P < 0.05, **P < 0.01, two-way ANOVA/Bonferroni. The application of laser light to GlyT2cre but not to Gad65cre mice running on the wheel triggers speed decrease, and higher laser intensities have a stronger impact. h, Representative single trial and group data speed analysis in the open field arena for optogenetic activation of GiA-GlyT2 (left), GiV-GlyT2 (middle) and Gi-vGAT (right) neurons 1 s before and during laser application. **P < 0.01, ***P < 0.001, unpaired t-test. Data are mean ± s.e.m., for sample sizes, see Supplementary Table 1.
Extended Data Figure 7 Fos expression levels in Mc subdomains and Gi after treadmill locomotion.
a, Schematic depiction of experimental strategy. Cage control mice are compared to mice running on a treadmill for 30 min, after which they are left undisturbed in the home cage before termination of the experiment and analysis of Fos expression. b, Representative example for Fos expression in cage control (left) compared to run (right) mice shown at similar rostro-caudal brainstem level at which optogenetic stimulation of LPGi-vGlut2 neurons elicits locomotion. c, Quantification of Fos-expressing neurons normalized to NeuN-expressing neurons on the same sections demonstrates a higher percentage of neurons in Mc subregions and Gi upon running. **P < 0.01, ***P < 0.001, unpaired t-test. d, e, Quantification of Fos intensity of analysed neurons, showing example neurons with corresponding pixel average values (d; left), and the percentage of Fos neurons with intensity values greater than 2,000, normalized to NeuNON neuronal number (d, right; n as in c; ***P < 0.001, two-way ANOVA/Bonferroni), and frequency distribution of Fos expression for different caudal brainstem regions analysed (e). dLPGi, dorsal LPGi; vLPGi, ventral LPGi. The cut-off used for the bar plot in d is shown in magenta (see Methods). Data are mean ± s.e.m., for sample sizes see Supplementary Table 1.
Extended Data Figure 8 Injection site location for loss-of-function experiments.
a–c, Analysis of injection positions targeting LPGi (a, b) or GiA (c) for loss-of-function experiments. b, For quantification of ablation efficiency, we used the injection of an AAV-flex marker (known to target most vGlut2-expressing neurons at the injection site) in mice without (left) compared to with (middle) coinjection of AAV-flex-DTR two weeks after DTA application. Note that 96.9% of targeted neurons are ablated with this strategy compared to corresponding reference control injections (right). Crosses in panels a and c depict centre of injections on brain atlas sections aligning with sites identified in corresponding experiments, with each colour representing a different mouse included in the analysis shown in Fig. 3. Data are mean ± s.e.m., for sample sizes see Supplementary Table 1. The mouse brain atlas images in this figure (a, c) have been reproduced with permission from Elsevier.
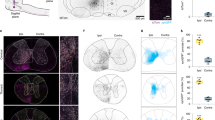
Extended Data Figure 9 Input and output circuitry of LPGi subpopulations.
a, b, Representative spinal cord sections depicting SynTag and ChAT labelling from LPGi-vGlut2 (a) or LPGi-GlyT2 (b) neurons for motor neurons (top), synaptic density analysis and white matter axon tract position (middle), and quantification of ipsilateral and contralateral synapse and axon fractions (bottom) at caudal cervical spinal cord levels. Percentages for ipsilateral spinal cords shown in the pie charts are as follows: LPGi-vGlut2 (SynTag: 59.97 ± 0.64; white matter axons: 74.80 ± 0.96), and LPGi-GlyT2 (SynTag: 54.15 ± 1.55; white matter axons: 79.21 ± 1.69). c, Schematic diagram of experimental strategy to map synaptic input to spinally projecting LPGi-vGlut2 and LPGi-GlyT2 neurons in vGlut2cre and GlyT2cre mice. NT, neurotransmitter. In a first injection, LPGi neurons are infected retrogradely from the spinal cord by a retro-AAV-flex-FLP, and locally by coinjection of AAV-ConFon-TVA and AAV-flex-G. In a second injection, LPGi is injected locally with EnvA-rabies-FP. d, e, Distribution of neurons within the MLR connected to LPGi-vGlut2 neurons. d, Images show example pictures of CnF neurons (not co-expressing ChAT) and PPN neurons, a minority of which is cholinergic (pie chart in e; percentage: 21.8 ± 7.2). e, Representative distribution of visualized neurons in CnF and PPN neurons overlaid on atlas section. f, Quantification demonstrating connectivity bias of MLR neurons to LPGi-vGlut2 compared with LPGi-GlyT2 neurons. *P < 0.05, unpaired t-test. Data are mean ± s.e.m., for sample sizes see Supplementary Table 1. The mouse brain atlas image in this figure has been reproduced with permission from Elsevier.
Extended Data Figure 10 Glutamatergic LPGi neurons mediate MLR motor commands.
a, b, Representative injection site (a) and projection pattern (b) of MLR-vGlut2 neurons labelled with SynTag. c, Single plane image of a spinally projecting vLPGi-vGlut2 dendrite with opposing MLR-vGlut2 synapses. d, e, Analysis of injection and corresponding optic fibre positions performed to optogenetically stimulate MLR-vGlut2 neurons (d, left) or their synaptic terminals in the Mc (d, right), and to determine the effect of LPGi-vGlut2 neuron ablation in response to MLR-vGlut2 light-induced locomotion (e), shown on brain atlas sections aligning with sites identified in corresponding experiments. Crosses depict centre of injections and rectangles show optic fibre tip positions, with each colour representing a different mouse included in the analysis shown in Fig. 4 and in g–j. f, Experimental scheme to stimulate MLR-vGlut2 neurons or their synaptic terminals optogenetically in the Mc using two optic fibres. g, Single trial speed analysis of stationary mouse for 1 s before laser onset (orange) compared to 1 s during laser application (cyan) for stimulations in the MLR (left) and terminals over the Mc (right). ***P < 0.001, paired t-test. h, Correlation between laser power and evoked speed upon stimulation of MLR-vGlut2 cell bodies (magenta) or axon terminals (cyan) over the Mc (different symbols indicate different mice). Note that cell body stimulation elicits stronger locomotor responses, but in both cases, higher laser intensities elicit higher speed locomotion (least square linear regression through origin followed by extra sum-of-squares F-test comparison; MLR slope: 42.41 ± 9.60, Mc slope: 3.70 ± 0.66, P < 0.0001). i, Speed versus time traces for single trials (grey lines) and average (dotted black line) of one representative mouse. j, Histogram of latency to start (left), duration of individual locomotor bouts (middle), and latency to stop (right) induced by MLR-vGlut2 neuron stimulation at the minimum laser intensity needed to evoke maximum reliability. Data are mean ± s.e.m., for sample sizes see Supplementary Table 1. The mouse brain atlas images in this figure have been reproduced with permission from Elsevier.
Supplementary information
Supplementary Information
This file contains additional discussion to the work presented in this study. It also contains Supplementary Table 1 which shows detailed statistical tests and sample sizes used in this study described in the accompanying main and extended data figures. (PDF 683 kb)
Stimulation of excitatory neurons in the caudal brainstem
Representative examples of video sequences showing the behavioral effects of mice in the open field arena upon laser application to LPGi-vGlut2, GiA-vGlut2, GiV-vGlut2, and Gi-vGlut2 neurons. (MP4 23824 kb)
Stimulation of inhibitory LPGi neuron subpopulations
Representative examples of video sequences showing the behavioral effects of mice in the open field arena upon laser application to LPGi-vGAT, LPGi-GlyT2 and LPGi-Gad65 neurons. (MP4 6267 kb)
Stimulation of glycinergic GiA, GiV and Gi neuron subpopulations
Representative examples of video sequences showing the behavioral effects of mice in the open field arena upon laser application to GiA-GlyT2, GiV-GlyT2 and Gi-vGAT neurons. (MP4 8811 kb)
Rights and permissions
About this article
Cite this article
Capelli, P., Pivetta, C., Soledad Esposito, M. et al. Locomotor speed control circuits in the caudal brainstem. Nature 551, 373–377 (2017). https://doi.org/10.1038/nature24064
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature24064
This article is cited by
-
Functional plasticity of glutamatergic neurons of medullary reticular nuclei after spinal cord injury in mice
Nature Communications (2024)
-
Basal ganglia–spinal cord pathway that commands locomotor gait asymmetries in mice
Nature Neuroscience (2024)
-
Multimodal cortical neuronal cell type classification
Pflügers Archiv - European Journal of Physiology (2024)
-
The mesencephalic locomotor region recruits V2a reticulospinal neurons to drive forward locomotion in larval zebrafish
Nature Neuroscience (2023)
-
How the brain plays musical statues
Nature Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.