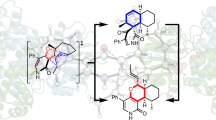
Abstract
Pericyclic reactions—which proceed in a concerted fashion through a cyclic transition state—are among the most powerful synthetic transformations used to make multiple regioselective and stereoselective carbon–carbon bonds1. They have been widely applied to the synthesis of biologically active complex natural products containing contiguous stereogenic carbon centres2,3,4,5,6. Despite the prominence of pericyclic reactions in total synthesis, only three naturally existing enzymatic examples (the intramolecular Diels–Alder reaction7, and the Cope8 and the Claisen rearrangements9) have been characterized. Here we report a versatile S-adenosyl-l-methionine (SAM)-dependent enzyme, LepI, that can catalyse stereoselective dehydration followed by three pericyclic transformations: intramolecular Diels–Alder and hetero-Diels–Alder reactions via a single ambimodal transition state, and a retro-Claisen rearrangement. Together, these transformations lead to the formation of the dihydropyran core of the fungal natural product, leporin10. Combined in vitro enzymatic characterization and computational studies provide insight into how LepI regulates these bifurcating biosynthetic reaction pathways by using SAM as the cofactor. These pathways converge to the desired biosynthetic end product via the (SAM-dependent) retro-Claisen rearrangement catalysed by LepI. We expect that more pericyclic biosynthetic enzymatic transformations remain to be discovered in naturally occurring enzyme ‘toolboxes’11. The new role of the versatile cofactor SAM is likely to be found in other examples of enzyme catalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoffmann, R. & Woodward, R. B. The conservation of orbital symmetry. Acc. Chem. Res. 1, 17–22 (1968)
Takao, K., Munakata, R. & Tadano, K. Recent advances in natural product synthesis by using intramolecular Diels-Alder reactions. Chem. Rev. 105, 4779–4807 (2005)
Nicolaou, K. C., Snyder, S. A., Montagnon, T. & Vassilikogiannakis, G. The Diels-Alder reaction in total synthesis. Angew. Chem. Int. Ed. 41, 1668–1698 (2002)
Ilardi, E. A., Stivala, C. E. & Zakarian, A. [3,3]-Sigmatropic rearrangements: recent applications in the total synthesis of natural products. Chem. Soc. Rev. 38, 3133–3148 (2009)
Ardkhean, R. et al. Cascade polycyclizations in natural product synthesis. Chem. Soc. Rev. 45, 1557–1569 (2016)
Nicolaou, K. C., Vourloumis, D., Winssinger, N. & Baran, P. S. The art and science of total synthesis at the dawn of the twenty-first century. Angew. Chem. Int. Ed. 39, 44–122 (2000)
Kim, H. J., Ruszczycky, M. W., Choi, S. H., Liu, Y. N. & Liu, H. W. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A. Nature 473, 109–112 (2011)
Li, S. et al. Hapalindole/ambiguine biogenesis is mediated by a Cope rearrangement, C–C bond-forming cascade. J. Am. Chem. Soc. 137, 15366–15369 (2015)
Andrews, P. R., Smith, G. D. & Young, I. G. Transition-state stabilization and enzymic catalysis. Kinetic and molecular orbital studies of the rearrangement of chorismate to prephenate. Biochemistry 12, 3492–3498 (1973)
Cary, J. W. et al. An Aspergillus flavus secondary metabolic gene cluster containing a hybrid PKS-NRPS is necessary for synthesis of the 2-pyridones, leporins. Fungal Genet. Biol. 81, 88–97 (2015)
Walsh, C. T. A chemocentric view of the natural product inventory. Nat. Chem. Biol. 11, 620–624 (2015)
Lin, C.-I., McCarty, R. M. & Liu, H.-W. The enzymology of organic transformations: a survey of name reactions in biological systems. Angew. Chem. Int. Ed. 56, 3446–3489 (2017)
Minami, A. & Oikawa, H. Recent advances of Diels-Alderases involved in natural product biosynthesis. J. Antibiot. 69, 500–506 (2016)
Tang, M.-C., Zou, Y., Watanabe, K., Walsh, C. T. & Tang, Y. Oxidative cyclization in natural product biosynthesis. Chem. Rev. 117, 5226–5333 (2017)
Stocking, E. M . & Williams, R. M. Chemistry and biology of biosynthetic Diels-Alder reactions. Angew. Chem. Int. Ed. 42, 3078–3115 (2003)
Oikawa, H. & Tokiwano, T. Enzymatic catalysis of the Diels-Alder reaction in the biosynthesis of natural products. Nat. Prod. Rep. 21, 321–352 (2004)
Desimoni, G. & Tacconi, G. Heterodiene syntheses with α,β-unsaturated carbonyl compounds. Chem. Rev. 75, 651–692 (1975)
Jessen, H. J. & Gademann, K. 4-Hydroxy-2-pyridone alkaloids: structures and synthetic approaches. Nat. Prod. Rep. 27, 1168–1185 (2010)
Van De Water, R. W. & Pettus, T. R. R. o-Quinone methides: intermediates underdeveloped and underutilized in organic synthesis. Tetrahedron 58, 5367–5405 (2002)
Snider, B. B. & Lu, Q. Total synthesis of (±)-leporin A. J. Org. Chem. 61, 2839–2844 (1996)
Li, L. et al. Biochemical characterization of a eukaryotic decalin-forming Diels–Alderase. J. Am. Chem. Soc. 138, 15837–15840 (2016)
Halo, L. M. et al. Late stage oxidations during the biosynthesis of the 2-pyridone tenellin in the entomopathogenic fungus Beauveria bassiana. J. Am. Chem. Soc. 130, 17988–17996 (2008)
Jansson, A. et al. Aclacinomycin 10-hydroxylase is a novel substrate-assisted hydroxylase requiring S-adenosyl-L-methionine as cofactor. J. Biol. Chem. 280, 3636–3644 (2005)
Jiang, C. et al. Formation of the Δ18,19 double bond and bis(spiroacetal) in salinomycin is atypically catalyzed by SlnM, a methyltransferase-like enzyme. Angew. Chem. Int. Ed. 54, 9097–9100 (2015)
Iwig, D. F. & Booker, S. J. Insight into the polar reactivity of the onium chalcogen analogues of S-adenosyl-L-methionine. Biochemistry 43, 13496–13509 (2004)
Coward, J. K. & Slisz, E. P. Analogs of S-adenosylhomocysteine as potential inhibitors of biological transmethylation. Specificity of the S-adenosylhomocysteine binding site. J. Med. Chem. 16, 460–463 (1973)
Bauer, N. J., Kreuzman, A. J., Dotzlaf, J. E. & Yeh, W. K. Purification, characterization, and kinetic mechanism of S-adenosyl-L-methionine:macrocin O-methyltransferase from Streptomyces fradiae. J. Biol. Chem. 263, 15619–15625 (1988)
Patel, A. et al. Dynamically complex [6+4] and [4+2] cycloadditions in the biosynthesis of spinosyn A. J. Am. Chem. Soc. 138, 3631–3634 (2016)
Ess, D. H. et al. Bifurcations on potential energy surfaces of organic reactions. Angew. Chem. Int. Ed. 47, 7592–7601 (2008)
Hong, Y. J. & Tantillo, D. J. Biosynthetic consequences of multiple sequential post-transition-state bifurcations. Nat. Chem. 6, 104–111 (2014)
Xu, W., Cai, X., Jung, M. E. & Tang, Y. Analysis of intact and dissected fungal polyketide synthase-nonribosomal peptide synthetase in vitro and in Saccharomyces cerevisiae. J. Am. Chem. Soc. 132, 13604–13607 (2010)
Frisch, M. J. et al. Gaussian 09 Rev. C.01 (Gaussian Inc., 2010)
Acknowledgements
This work was supported by the NIH (1DP1GM106413 and 1R35GM118056), the NSF (CHE-1361104 to K.N.H.), and the JSPS Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (G2604 to K.W.).
Author information
Authors and Affiliations
Contributions
M.O., F.L., Y.H., K.N.H. and Y.T. developed the hypothesis and designed the study. M.O. performed all in vivo and in vitro experiments, as well as compound isolation and characterization. M.O., Y.H. and M.C. performed protein purification. M.S. and M.-C.T. performed compound characterization. F.L., Z.Y. and K.N.H. performed the computational experiments. All authors analysed and discussed the results. M.O., F.L., K.W., K.N.H. and Y.T. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 LC–MS analysis of the in vitro reaction of 3 with LepF.
The extracted ion chromatograms (EIC) under positive ionization are shown. The mass/charge (m/z) ratio of alcohols 4 and 4′ is 354 under positive ionization. Because the enzymatic activity of LepF is low and 4 is very unstable, we were not able to obtain enough 4 to use it as the substrate for in vitro reaction of LepI. Thus, we obtained 4 by reducing ketone 3 with NaBH4. Since this reduction proceeds non-stereoselectively, 4 and diastereomer 4′ were formed. After the isolation of 4 and 4′ by HPLC, the fractions containing 4 and 4′ were not concentrated and were immediately used as the substrate. The stereochemistry of the secondary alcohol in 4 and 4′ was not determined.
Extended Data Figure 2 HPLC analysis of the chemical reduction of 3 with NaBH4.
The reaction mixture containing 1 mM 3 and 10 mM NaBH4 with EtOH (50 μl) was incubated at 0 °C for 1 min, then the reaction was quenched with water. After centrifugation, the supernatant was analysed by HPLC. The reduction of 3 gave the alcohol 4 and diastereomer 4′. The spontaneous dehydration of both alcohols resulted in the formation of HDA and IMDA products via the E/Z mixture of quinone methide 5. The isolated 4 and 4′ also readily dehydrated and converted to a mixture of the desired HDA (2) and the undesired HDA (9) and IMDA (6–8) products, showing the instability of these compounds. The structures (right) show the relative stereochemistry.
Extended Data Figure 3 Reaction analysis of 6–9 under heating.
6 (dissolved in 5% DMSO with H2O) was heated at 95 °C for 1 h. 7–9 (dissolved in 5% DMSO with H2O) were heated at 95 °C for 10 h. 6 was completely converted to 2 via [3,3]-sigmatropic retro-Claisen rearrangement. This reaction is irreversible under these conditions. It should be noted that the conversion of 6 to 2 via cycloreversion can be ruled out, since 6 was completely converted to 2 without any other IMDA/HDA side products. No reactions occurred in the case of 7. 8 and 9 can be interconverted via Claisen rearrangement. In this case, retro-Claisen rearrangement (8 to 9) is preferable to forward Claisen rearrangement (9 to 8). The structures show the relative stereochemistry.
Extended Data Figure 4 Analysis of the substrate specificity of LepI.
a, In vitro reactions of other IMDA products 7–9 with 30 μM LepI for 12 h. (i) 8 in buffer, (ii) 8 with LepI, (iii) 7 in buffer, (iv) 7 with LepI, (v) 9 in buffer, (vi) 9 with LepI. The experimental details are described in Methods. b, Elucidation of inhibitory activity of 7 on LepI-catalysed retro-Claisen rearrangement of 6 to 2. The experimental details are described in Methods. The IC50 value is mean ± standard deviation (s.d.) of three independent experiments. The structures show the relative stereochemistry.
Extended Data Figure 5 Time-course analysis of the LepI-catalysed retro-Claisen rearrangement of 6 to 2.
The experimental details are described in Methods. The data show one representative experiment from at least three independent replicates.
Extended Data Figure 6 HPLC analysis showing that purified LepI retains SAM.
SAM was detected in the supernatant of denatured (by acetonitrile) LepI. When LepI was denatured by heating the sample at 95 °C for 10 min, a single peak corresponding to 5′-deoxy-5′-(methylthio)adenosine (MTA), a major degradation product of SAM25, was detected from the supernatant of boiled LepI. Since SAM to MTA conversion is nearly quantitative and an MTA standard curve can be readily constructed, we found that about 90% of LepI still retains SAM after purification. Shown are HPLC profiles of (i) LepI denatured by acetonitrile, (ii) LepI heated at 95 °C for 10 min, (iii) the authentic reference of SAM, (iv) SAM heated at 95 °C for 10 min, and (v) the authentic reference of MTA. The experimental details are described in Methods.
Extended Data Figure 7 HPLC analysis of SAM-dependent LepI-catalysed reactions.
a, Analysis of in vitro reaction of 240 μM 4 with 300 nM LepI at 30 °C for 5 min in the presence and absence of cofactors. The concentrations of SAH, SAM and sinefungin used in this experiment are 250 μM, 100 μM and 100 μM, respectively. The data show one representative experiment from at least three independent replicates. b, Analysis of the in vitro reaction of 140 μM 6 with 300 nM LepI at 30 °C for 4 min in the presence and absence of cofactors. The concentrations of SAH, SAM and sinefungin used in this experiment are 250 μM, 100 μM and 100 μM, respectively. The data show one representative experiment from at least three independent replicates.
Extended Data Figure 8 SAH is a competitive inhibitor of LepI retro-Claisen rearrangement.
a, Dose-dependent inhibition of retro-Claisen rearrangement by SAH. b, Dose-dependent recovery of retro-Claisen rearrangement by SAM in the presence of 250 μM SAH. The experimental details are described in Methods. Error bars, s.d. of three independent experiments.
Extended Data Figure 9 Time-course analysis of the production of 2 divided by the sum of the production of 2 and 6.
The substrate used in this study is alcohol 4. a, LepI-catalysed reaction with or without SAH (250 μM). b, Non-enzymatic reaction. The initial production ratio (IMDA (6) versus HDA products (2)) between LepI-catalysed (about 1:1 periselectivity) and non-catalysed reactions (about 94:6 periselectivity) are clearly different. These data support the suggestion that LepI catalyses the competitive IMDA/HDA reactions by changing the product distribution resulting from IMDA versus HDA reactions.
Extended Data Figure 10 Calculated free energies and bond distances.
Data are shown for the ambimodal transition state (TS-1) and the transition state for the retro-Claisen rearrangement (TS-2), uncatalysed and with various catalysts, calculated with B3LYP-D3/6-311+G(d,p)//6-31G(d), CPCM water. Positions of the bonds are shown in the structures above.
Supplementary information
Supplementary Information
This file contains Supplementary Methods 1-4, Supplementary Tables 1-9, Supplementary Figures 1-32, Supplementary Data and additional references. (PDF 17133 kb)
Rights and permissions
About this article
Cite this article
Ohashi, M., Liu, F., Hai, Y. et al. SAM-dependent enzyme-catalysed pericyclic reactions in natural product biosynthesis. Nature 549, 502–506 (2017). https://doi.org/10.1038/nature23882
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23882
This article is cited by
-
The evolutionary origin of naturally occurring intermolecular Diels-Alderases from Morus alba
Nature Communications (2024)
-
A cyclase that catalyses competing 2 + 2 and 4 + 2 cycloadditions
Nature Chemistry (2023)
-
O-methyltransferase-like enzyme catalyzed diazo installation in polyketide biosynthesis
Nature Communications (2023)
-
Genome mining for unknown–unknown natural products
Nature Chemical Biology (2023)
-
An NmrA-like enzyme-catalysed redox-mediated Diels–Alder cycloaddition with anti-selectivity
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.