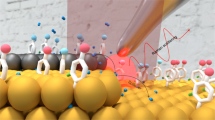
Abstract
The activity of heterogeneous catalysts—which are involved in some 80 per cent of processes in the chemical and energy industries—is determined by the electronic structure of specific surface sites that offer optimal binding of reaction intermediates. Directly identifying and monitoring these sites during a reaction should therefore provide insight that might aid the targeted development of heterogeneous catalysts and electrocatalysts (those that participate in electrochemical reactions) for practical applications. The invention of the scanning tunnelling microscope (STM)1,2 and the electrochemical STM3,4 promised to deliver such imaging capabilities, and both have indeed contributed greatly to our atomistic understanding of heterogeneous catalysis5,6,7,8. But although the STM has been used to probe and initiate surface reactions9,10, and has even enabled local measurements of reactivity in some systems11,12,13, it is not generally thought to be suited to the direct identification of catalytically active surface sites under reaction conditions. Here we demonstrate, however, that common STMs can readily map the catalytic activity of surfaces with high spatial resolution: we show that by monitoring relative changes in the tunnelling current noise, active sites can be distinguished in an almost quantitative fashion according to their ability to catalyse the hydrogen-evolution reaction or the oxygen-reduction reaction. These data allow us to evaluate directly the importance and relative contribution to overall catalyst activity of different defects and sites at the boundaries between two materials. With its ability to deliver such information and its ready applicability to different systems, we anticipate that our method will aid the rational design of heterogeneous catalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Binnig, G., Rohrer, H., Gerber, C. & Weibel, E. Surface studies by scanning tunneling microscopy. Phys. Rev. Lett. 49, 57–61 (1982)
Binnig, G., Rohrer, H., Gerber, Ch. & Weibel, E. Tunneling through a controllable vacuum gap. Appl. Phys. Lett. 40, 178–180 (1982)
Liu, H.-Y., Fan, F.-R. F., Lin, C. W. & Bard, A. J. Scanning electrochemical and tunneling ultramicroelectrode microscope for high-resolution examination of electrode surfaces in solution. J. Am. Chem. Soc. 108, 3838–3839 (1986)
Itaya, K. & Tomita, E. Scanning tunneling microscope for electrochemistry—a new concept for the in situ scanning tunneling microscope in electrolyte solutions. Surf. Sci. 201, L507–L512 (1988)
Thomas, J. M. & Thomas, W. J. Principles and Practice of Heterogeneous Catalysis 2nd edn (John Wiley, 2014)
Chorkendorff, I. & Niemantsverdriet, J. W. Concepts of Modern Catalysis and Kinetics (John Wiley, 2006)
Bell, T. A. The impact of nanoscience on heterogeneous catalysis. Science 299, 1688–1691 (2003)
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007)
Nørskov, J. K. Surface chemistry: catalysis frozen in time. Nature 414, 405–406 (2001)
Hahn, J. & Ho, W. Oxidation of a single carbon monoxide molecule manipulated and induced with a scanning tunneling microscope. Phys. Rev. Lett. 87, 166102 (2001)
Zambelli, T., Wintterlin, J., Trost, J. & Ertl, G. Identification of the “active sites” of a surface-catalyzed reaction. Science 273, 1688–1690 (1996)
Meier, J., Friedrich, K. A. & Stiming, U. Novel method for the investigation of single nanoparticle reactivity. Faraday Discuss. 121, 365–372 (2002)
Wolfschmidt, H., Weingarth, D. & Stimming, U. Enhanced reactivity for hydrogen reactions at Pt nanoislands on Au(111). ChemPhysChem 11, 1533–1541 (2010)
Möller, R., Esslinger, A. & Koslowski, B. Noise in vacuum tunneling: application for a novel scanning microscope. Appl. Phys. Lett. 55, 2360–2362 (1989)
Hugelmann, M. & Schindler, W. Tunnel barrier height oscillations at the solid/liquid interface. Surf. Sci. 541, L643–L648 (2003)
Koper, M. T. M. Structure sensitivity and nanoscale effects in electrocatalysis. Nanoscale 3, 2054–2073 (2011)
Tymoczko, J., Calle-Vallejo, F., Schuhmann, W. & Bandarenka, A. S. Making the hydrogen evolution reaction in PEM electrolyzers even faster. Nat. Commun. 7, 10990 (2016)
Perez, J., Gonzalez, E. R. & Villullas, H. M. Hydrogen evolution reaction on gold single-crystal electrodes in acid solutions. J. Phys. Chem. B 102, 10931–10935 (1998)
Pandelov, S. & Stimming, U. Reactivity of monolayers and nano-islands of palladium on Au(111) with respect to proton reduction. Electrochim. Acta 52, 5548–5555 (2007)
Kibler, L. A. Hydrogen electrocatalysis. ChemPhysChem 7, 985–991 (2006)
Hernandez, F. & Baltruschat, H. Hydrogen evolution and Cu UPD at stepped gold single crystals modified with Pd. J. Solid State Electrochem. 11, 877–885 (2007)
Björketun, M. E. et al. Hydrogen evolution on Au(111) covered with submonolayers of Pd. Phys. Rev. B 84, 045407 (2011)
Stephens, I. E. L., Bondarenko, A. S., Grønbjerg, U., Rossmeisl, J. & Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 5, 6744–6762 (2012)
Battino, R. (ed.) Oxygen and Ozone: Solubility Data Series Vol. 7 (Pergamon, 1981)
Takesue, Y., Nakamura, M. & Hoshi, N. Structural effects on the oxygen reduction reaction on the high index planes of Pt3Co. Phys. Chem. Chem. Phys. 16, 13774–13779 (2014)
Rurigaki, T., Hitotsuyanagi, A., Nakamura, M., Sakai, N. & Hoshi, N. Structural effects on the oxygen reduction reaction on the high index planes of Pt3Ni: n(111)–(111) and n(111)–(100) surfaces. J. Electroanal. Chem. 716, 58–62 (2014)
Kuzume, A., Herrero, E. & Feliu, J. M. Oxygen reduction on stepped platinum surfaces in acidic media. J. Electroanal. Chem. 599, 333–343 (2007)
Hitotsuyanagi, A., Nakamura, M. & Hoshi, N. Structural effects on the activity for the oxygen reduction reaction on n(111)–(100) series of Pt: correlation with the oxide film formation. Electrochim. Acta 82, 512–516 (2012)
Bandarenka, A. S., Hansen, H. A., Rossmeisl, J. & Stephens, I. E. L. Elucidating the activity of stepped Pt single crystals for oxygen reduction. Phys. Chem. Chem. Phys. 16, 13625–13629 (2014)
Calle-Vallejo, F. et al. Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors. Science 350, 185–189 (2015)
Horcas, I. et al. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705 (2007)
Kibler, L., Cuesta, A., Kleinert, M. & Kolb, D. In-situ STM characterisation of the surface morphology of platinum single crystal electrodes as a function of their preparation. J. Electroanal. Chem. 484, 73–82 (2000)
Čolić, V. et al. Experimental aspects in benchmarking of the electrocatalytic activity. ChemElectroChem 2, 143–149 (2015)
Liu, Y., Gokcen, D., Bertocci, U. & Moffat, T. P. Self-terminating growth of platinum films by electrochemical deposition. Science 338, 1327–1330 (2012)
Brankovic, S., Wang, J. & Adžic´, R. Metal monolayer deposition by replacement of metal adlayers on electrode surfaces. Surf. Sci. 474, L173–L179 (2001)
Reiss, G., Schneider, F., Vancea, J. & Hoffmann, H. Scanning tunneling microscopy on rough surfaces: deconvolution of constant current images. Appl. Phys. Lett. 57, 867–869 (1990)
Trasatti, S. & Petrii, O. Real surface area measurements in electrochemistry. Pure Appl. Chem. 63, 711–734 (1991)
Zhang, L. et al. Electrochemically controlled formation and growth of hydrogen nanobubbles. Langmuir 22, 8109–8113 (2006)
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG; project BA 5795/3-1) and the cluster of excellence Nanosystems Initiative Munich (NIM) for financial support. O.S. acknowledges funding from Toyota Motor Europe.
Author information
Authors and Affiliations
Contributions
J.H.K.P. and Y.L. conducted the experiments, performed the data analysis and contributed to manuscript preparation. A.S.B. developed the basic idea, wrote large parts of the manuscript and supervised J.H.K.P. and Y.L. in their experimental work. O.S. suggested the use of the palladium/gold system, contributed background knowledge, advised Y.L. in his experimental work and contributed to the manuscript-drafting process. All the authors participated in discussing the results and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks S. Boettcher, P. Davies and J. Kunze-Liebhäuser for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Typical cyclic voltammogram for an insulated Pt/Ir STM tip.
The potentials of the working electrode were set such that no HER was taking place. The desorption peak of hydrogen at around −1.2 V versus Au/AuOx (see expanded area) can be used to determine the exposed area at the apex of the tip.
Extended Data Figure 2 Cyclic voltammograms of Pt(111) and Au(111).
a, Typical cyclic voltammogram for a Pt(111) single crystal in 0.1 M HClO4 after flame-annealing, revealing an increased amount of (step-like) defects compared with a ‘perfect’ Pt(111) crystal. b, Typical cyclic voltammogram for an Au(111) single crystal in 1 M HClO4 after annealing.
Extended Data Figure 3 Pt deposition on Au.
a, Linear sweep voltammetry scan of a test Au sample, for determining the deposition potential in 0.5 M NaCl and 3 mM K2PtCl6 at a scan rate of 10 mV s−1. The arrow indicates the scan direction. b, Deposition-current transients on a fresh, annealed Au sample. c, Resulting cyclic voltammogram for Pt/Au in 0.5 M H2SO4 solution at a scan rate of 20 mV s−1.
Extended Data Figure 4 XPS data for an Au sample that is half covered by Pt.
a, Pt 4d 5/2 XPS peaks for the Pt-covered half of the sample (‘Pt position’) and the uncovered half (‘Au position’). b, Pt 4f 5/2 and 7/2 peaks for both halves of the sample.
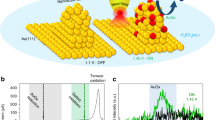
Extended Data Figure 5 EC-STM images of Pt and Au electrode surfaces in 0.1 M HClO4.
a–f, Sequential EC-STM images (constant-current mode) of roughly the same Pt electrode area; the electrode potential was initially kept to a value at which no hydrogen evolution was thermodynamically possible (‘OFF’), and then changed to enable HER on Pt (‘ON’). g−j, Electrochemical STM images for the Au electrode, obtained as for the Pt sample; the strong features of tunnelling current noise do not appear here, because the reaction is not catalysed by Au at this electrode potential.
Extended Data Figure 6 EC-STM images of Pt, obtained with HER ON or OFF.
a–f, Three-dimensional representations of a series of images recorded successively (following the blue arrows) over Pt deposited on an Au substrate (in 0.1 M HClO4) while switching the working-electrode potentials (halfway through each image) such that no hydrogen evolves (OFF) or hydrogen evolution takes place (ON). Alternating scanning directions are indicated by black arrows, starting with a downward scan in a, c, e, and an upward scan in b, d, f. Substantial increases in tunnelling noise are observed when the working-electrode potential enables HER (ON); no such noise features are visible for the OFF regions.
Extended Data Figure 7 EC-STM images of Au at different potentials.
a–d, Three-dimensional representations of images recorded successively over Au. Black arrows show scanning directions. The sample under investigation is an Au substrate, with one half of its surface covered with Pt and the other half left uncovered (see Extended Data Figs 5a–f and 6 for measurements on the Pt region). The sample potentials were switched (halfway through each image) such that no hydrogen evolves (OFF) or hydrogen evolution begins (ON) on the Pt side. Given that the required overpotential for the HER on Au is larger than that on Pt, there should be no hydrogen evolution at all on the Au side, leading to no notable change in tunnelling noise (in contrast with the situation in Extended Data Figs 5a–f and 6). Indeed, variations in tunnelling noise could not be detected in either direction (going from OFF to ON and vice versa). This also shows that the changed bias potential between ON and OFF does not substantially impact the recorded measurement noise.
Extended Data Figure 8 Potentiodynamic cyclic voltammogram for a Pt(111) single crystal.
This cyclic voltammogram was measured in air-saturated 0.1 M HClO4 electrolyte using a Pt pseudo-reference electrode. Scan rate, 20 mV s–1.
Extended Data Figure 9 Deposition of Pt nanoparticles and selectivity measurements.
a, Current transient taken during the deposition of Pt nanoparticles on an Au substrate. b, Cyclic voltammogram, measured after deposition, in 0.1 M HClO4 and at a scan rate of 20 mV s−1. c, EC-STM image of Pt nanoparticles on an Au substrate. The yellow parts are likely to be Pt nanoparticles, on top of protruding Au. d, Results of tip retraction during HER over Pt/Au, confirming the yellow parts in c to be the more-active Pt (compared with the less-active Au).
Extended Data Figure 10 Cu underpotential deposition.
a, Cyclic voltammogram for a thin film of quasi-Au(111) in a solution of 0.05 mM CuSO4 and 0.5 M H2SO4 at a scan rate of 20 mV s−1. b, Current transient taken during Cu UPD at −0.32 V versus MMS for 4 s.
Source data
Rights and permissions
About this article
Cite this article
Pfisterer, J., Liang, Y., Schneider, O. et al. Direct instrumental identification of catalytically active surface sites. Nature 549, 74–77 (2017). https://doi.org/10.1038/nature23661
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23661
This article is cited by
-
Site-specific reactivity of stepped Pt surfaces driven by stress release
Nature (2024)
-
Visualizing the structural evolution of individual active sites in MoS2 during electrocatalytic hydrogen evolution reaction
Nature Catalysis (2024)
-
Strain engineering in electrocatalysis: Strategies, characterization, and insights
Nano Research (2024)
-
Near ambient N2 fixation on solid electrodes versus enzymes and homogeneous catalysts
Nature Reviews Chemistry (2023)
-
Density Functional Calculations of the Sequential Adsorption of Hydrogen on Single Atom and Small Clusters of Pd and Pt Supported on Au(111)
Electrocatalysis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.