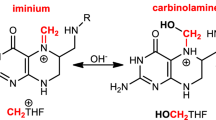
Abstract
The folate-driven one-carbon (1C) cycle is a fundamental metabolic hub in cells that enables the synthesis of nucleotides and amino acids and epigenetic modifications. This cycle might also release formaldehyde, a potent protein and DNA crosslinking agent that organisms produce in substantial quantities. Here we show that supplementation with tetrahydrofolate, the essential cofactor of this cycle, and other oxidation-prone folate derivatives kills human, mouse and chicken cells that cannot detoxify formaldehyde or that lack DNA crosslink repair. Notably, formaldehyde is generated from oxidative decomposition of the folate backbone. Furthermore, we find that formaldehyde detoxification in human cells generates formate, and thereby promotes nucleotide synthesis. This supply of 1C units is sufficient to sustain the growth of cells that are unable to use serine, which is the predominant source of 1C units. These findings identify an unexpected source of formaldehyde and, more generally, indicate that the detoxification of this ubiquitous endogenous genotoxin creates a benign 1C unit that can sustain essential metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tibbetts, A. S. & Appling, D. R. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 30, 57–81 (2010)
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2017)
Walport, L. J., Hopkinson, R. J. & Schofield, C. J. Mechanisms of human histone and nucleic acid demethylases. Curr. Opin. Chem. Biol. 16, 525–534 (2012)
Pontel, L. B. et al. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol. Cell 60, 177–188 (2015)
Heck, H. D. et al. Formaldehyde (CH2O) concentrations in the blood of humans and Fischer-344 rats exposed to CH2O under controlled conditions. Am. Ind. Hyg. Assoc. J. 46, 1–3 (1985)
Luo, W., Li, H., Zhang, Y. & Ang, C. Y. Determination of formaldehyde in blood plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 753, 253–257 (2001)
Nagy, K., Pollreisz, F., Takáts, Z. & Vékey, K. Atmospheric pressure chemical ionization mass spectrometry of aldehydes in biological matrices. Rapid Commun. Mass Spectrom. 18, 2473–2478 (2004)
Osborn, M. J., Talbert, P. T. & Huennekens, F. M. The structure of ‘active formaldehyde’ (N5, N10-methylene tetrahydrofolic acid). J. Am. Chem. Soc. 82, 4291–4297 (1960)
Horne, D. W. Neither methionine nor nitrous oxide inactivation of methionine synthase affect the concentration of 5,10-methylenetetrahydrofolate in rat liver. J. Nutr. 133, 476–478 (2003)
Scott, K. C. & Grogory, J. F., III . The fate of [3H]folic aicd in folate-adequate rats. J. Nutr. Biochem. 7, 261–269 (1996)
Schmitz, J. C., Grindey, G. B., Schultz, R. M. & Priest, D. G. Impact of dietary folic acid on reduced folates in mouse plasma and tissues. Relationship to dideazatetrahydrofolate sensitivity. Biochem. Pharmacol. 48, 319–325 (1994)
Rosado, I. V., Langevin, F., Crossan, G. P., Takata, M. & Patel, K. J. Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway. Nat. Struct. Mol. Biol. 18, 1432–1434 (2011)
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23, 1140–1153 (2016)
Patel, H., Pietro, E. D. & MacKenzie, R. E. Mammalian fibroblasts lacking mitochondrial NAD+-dependent methylenetetrahydrofolate dehydrogenase-cyclohydrolase are glycine auxotrophs. J. Biol. Chem. 278, 19436–19441 (2003)
McCarthy, E. A., Titus, S. A., Taylor, S. M., Jackson-Cook, C. & Moran, R. G. A mutation inactivating the mitochondrial inner membrane folate transporter creates a glycine requirement for survival of chinese hamster cells. J. Biol. Chem. 279, 33829–33836 (2004)
Lawrence, S. A. et al. Mammalian mitochondrial and cytosolic folylpolyglutamate synthetase maintain the subcellular compartmentalization of folates. J. Biol. Chem. 289, 29386–29396 (2014)
Meiser, J. et al. Serine one-carbon metabolism with formate overflow. Sci. Adv. 2, e1601273 (2016)
Chippel, D. & Scrimgeour, K. G. Oxidative degradation of dihydrofolate and tetrahydrofolate. Can. J. Biochem. 48, 999–1009 (1970)
Brewer, T. F. & Chang, C. J. An aza-Cope reactivity-based fluorescent probe for imaging formaldehyde in living cells. J. Am. Chem. Soc. 137, 10886–10889 (2015)
De Brouwer, V., Zhang, G. F., Storozhenko, S., Straeten, D. V. & Lambert, W. E. pH stability of individual folates during critical sample preparation steps in prevision of the analysis of plant folates. Phytochem. Anal. 18, 496–508 (2007)
Uotila, L. & Koivusalo, M. Formaldehyde dehydrogenase from human liver. Purification, properties, and evidence for the formation of glutathione thiol esters by the enzyme. J. Biol. Chem. 249, 7653–7663 (1974)
Uotila, L. & Koivusalo, M. Purification and properties of S-formylglutathione hydrolase from human liver. J. Biol. Chem. 249, 7664–7672 (1974)
Bae, S., Chon, J., Field, M. S. & Stover, P. J. Alcohol dehydrogenase 5 is a source of formate for de novo purine biosynthesis in HepG2 cells. J. Nutr. 147, 499–505 (2017)
Kownacki-Brown, P. A., Wang, C., Bailey, L. B., Toth, J. P. & Gregory, J. F., III . Urinary excretion of deuterium-labeled folate and the metabolite p-aminobenzoylglutamate in humans. J. Nutr. 123, 1101–1108 (1993)
Murphy, M. & Scott, J. M. The turnover catabolism and excretion of folate administered at physiological concentrations in the rat. Biochim. Biophys. Acta 583, 535–539 (1979)
Witham, K. L. et al. 5-methyl-tetrahydrofolate and the S-adenosylmethionine cycle in C57BL/6J mouse tissues: gender differences and effects of arylamine N-acetyltransferase-1 deletion. PLoS One 8, e77923 (2013)
Marean, A., Graf, A., Zhang, Y. & Niswander, L. Folic acid supplementation can adversely affect murine neural tube closure and embryonic survival. Hum. Mol. Genet. 20, 3678–3683 (2011)
Pickell, L. et al. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res. A Clin. Mol. Teratol. 91, 8–19 (2011)
Ghandour, H., Chen, Z., Selhub, J. & Rozen, R. Mice deficient in methylenetetrahydrofolate reductase exhibit tissue-specific distribution of folates. J. Nutr. 134, 2975–2978 (2004)
Farber, S. & Diamond, L. K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J. Med. 238, 787–793 (1948)
Nomura, Y., Adachi, N. & Koyama, H. Human Mus81 and FANCB independently contribute to repair of DNA damage during replication. Genes Cells 12, 1111–1122 (2007)
Wit, N. et al. Roles of PCNA ubiquitination and TLS polymerases κ and η in the bypass of methyl methanesulfonate-induced DNA damage. Nucleic Acids Res. 43, 282–294 (2015)
Garaycoechea, J. I. et al. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature 489, 571–575 (2012)
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013)
Bruemmer, K. J. et al. Development of a general aza-Cope reaction trigger applied to fluorescence imaging of formaldehyde in living cells. J. Am. Chem. Soc. 139, 5338–5350 (2017)
Arakawa, H., Lodygin, D. & Buerstedde, J. M. Mutant loxP vectors for selectable marker recycle and conditional knock-outs. BMC Biotechnol. 1, 7 (2001)
Acknowledgements
We thank C. Knox and the LMB mouse facility, the LMB FACS facility and G. MacKay and D. Sumpton from the Beatson Metabolomics Unit. We thank H. Koyama for providing us with the FANCB-deficient NALM-6 strain. We are grateful to T. Elliott and A. Liang for assistance with the LMB LC–MS analyses. G.B.B. was funded by the CRUK Cambridge Cancer Centre studentship and the Wellcome Trust. N.W. was funded by Children With Cancer and the Wellcome Trust. L.B.P. was funded by the Wellcome Trust. F.A.D. and L.M. were funded by CRUK. I.V.R. was funded by BFU2013-42918-P, AES15/01409 (CP12/03273), European Union (FEDER) and RYC-2015-18670. We thank the NIH (GM 79465 to C.J.C.) for support of this work. C.J.C. is an Investigator of the Howard Hughes Medical Institute. T.F.B. was partially supported by a Chemical Biology Training Grant from the NIH (T32 GM066698). R.L.C. and P.S.M. are supported by the MRC. R.L.C. is also supported by the EPSRC. J.M., M.P. and A.V. were funded by CRUK (C596/A21140 and C596/A18076). J.M. was also funded by a DFG Fellowship (ME 4636/2-1). K.J.P. is supported by the Medical Research Council, CRUK and the Wellcome Trust.
Author information
Authors and Affiliations
Contributions
G.B.B., N.W. and K.J.P. conceived the study. G.B.B. and N.W. conducted the majority of the experiments with assistance from F.A.D., L.M., L.B.P. and I.V.R. T.F.B. and C.J.C. provided the FAP-1 reagent and technical assistance. Metabolic analysis was conceived, performed and analysed mainly by J.M., M.P. and A.V with contributions from G.B.B., N.W., F.A.D., R.L.C. and P.S.M. K.J.P. wrote the manuscript with assistance from G.B.B. and N.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Cellular sensitivity of DT40 mutants to THF and formaldehyde.
a, Wild-type, ∆ADH5 and ∆FANCC chicken DT40 cells were exposed to doses of THF for 24 h. Western blots of lysates prepared from these cells were probed for FANCD2, CHK1 phosphorylation (CHK1-pS345) and β-actin. Uncropped scans can be seen in Supplementary Fig. 1. FANCD2-Ub, monoubiquitinated FANCD2. b, Cellular sensitivity of DT40 mutants in Fanconi anaemia core-complex genes (FANCB and FANCL) to THF and formaldehyde. c, Cellular sensitivity of other Fanconi-deficient DT40 mutants to THF and formaldehyde. d, Cellular sensitivity to THF and formaldehyde of a panel of DT40 mutant cell lines defective in different DNA repair pathways: Fanconi anaemia pathway (∆FANCB), translesion synthesis (∆REV3), nucleotide excision repair (∆XPA), homologous recombination (∆XRCC2) and non-homologous end joining (∆KU70). e, Top, scheme showing the reaction of formaldehyde with β-mercaptoethanol (β-ME). Bottom, suppression of THF and formaldehyde toxicity in wild-type, ∆ADH5 and ∆FANCC DT40 cells by the addition of 100 μM β-ME. Data in b–e are from three independent experiments, each carried out in triplicate, and represent mean ± s.e.m.
Extended Data Figure 2 THF toxicity in human cell lines deficient in protection against formaldehyde.
a, CRISPR–Cas9-mediated disruptions of the ADH5 locus in the human B cell line NALM-6. Exon 3 was targeted (in red) and the genetic changes (of one clone) in the sequence of both alleles are shown below. b, Western blot analysis of NALM-6 targeted clones for ADH5. Uncropped scans can be seen in Supplementary Fig. 1. c, Cellular sensitivity of wild-type, ∆ADH5 and ∆FANCB NALM-6 strains to THF and formaldehyde. Four ∆ADH5 clones were used. d, Suppression of THF and formaldehyde toxicity in wild-type and ∆ADH5 HAP1 cells by the addition of 100 μM β-ME. Data in c and d are from three independent experiments, each carried out in triplicate, and represent mean ± s.e.m.
Extended Data Figure 3 Characterization and THF sensitivity of a panel of 1C-cycle-deficient HAP1-mutant cell lines.
a, Scheme depicting key elements of the 1C cycle. The possible release of genotoxic formaldehyde (red) is shown. The enzymes targeted in HAP1 in this study are shown in bold. mTHF, monoglutamated THF (inactive); pTHF, polyglutamated THF (active); dTMP, deoxythymidine monophosphate. b, Metabolic characterization of the usage of [U-13C]serine in the 1C cycle by HAP1 mutants deficient in the proteins shown in a. The mass isotopomer distributions of glycine, formate and ATP are shown. Data are from two triplicate experiments. c, Functional characterization of the same HAP1 mutant strains by growth in media with (+) or without (–) glycine, HT and formate. Data represent two independent experiments, each with six replicates. d–f, Cellular sensitivity of the same cell lines to THF. Data are from two independent experiments, each done in triplicate. Three independent clones were used for the 1C-cycle-deficient mutant strains. All data represent mean ± s.e.m.
Extended Data Figure 4 Strategy, genetic characterization and protein expression of CRISPR–Cas9-mediated knockouts in HAP1.
a, Scheme outlining the sequential strategy used to generate a panel of CRISPR–Cas9-mediated knockout cell lines in HAP1. The double nickase approach (pX461) was used to generate mutants that were subsequently targeted. Wild-type Cas9 (pX458) was used for the other targeting events. b, Targeted exons (red) and genetic changes (in one clone) of the 1C cycle genes disrupted in HAP1 in this study. c, Western blot analysis for ADH5, SHMT1 and SHMT2 expression in the HAP1 strains used in this study. Uncropped scans can be seen in Supplementary Fig. 1.
Extended Data Figure 5 Oxidative degradation of folate derivatives liberates formaldehyde.
a, In vitro activation of the FAP-1 probe by a concentration range of formaldehyde or THF. Formaldehyde and THF were incubated in PBS at 37 °C for 24 h before the addition of FAP-1 and the measurement of fluorescence. b, FAP-1 activation by formaldehyde in the absence or presence of ascorbate or β-mercaptoethanol (β-ME). c, Release of formaldehyde by THF (400 μM) in PBS at different temperatures over time, measured using FAP-1. t1/2, estimated time required to release 50% of the total formaldehyde. Data in a–c are from two duplicate experiments. d, Top, detection of intracellular formaldehyde by FAP-1. Bottom, flow cytometry plot showing the fluorescence intensity of HAP1 cells. e, Activation of intracellular FAP-1 in HAP1 cells by formaldehyde or THF in the presence of a concentration range of ascorbate. f, Activation of FAP-1 in HAP1 cells exposed to folate derivatives sensitive or resistant to oxidative degradation. Data in e and f are from three duplicate experiments. A detailed description of the flow cytometry analysis in d–f can be seen in Supplementary Fig. 2. g, Measurement by gas chromatography–mass spectrometry (GC–MS) of the formaldehyde released by folate derivatives (200 μM) after 24 h incubation in PBS at 37 °C. In the case of the oxidation-resistant derivatives, formaldehyde was not detected (n.d.) (limit of detection: 1.66 μM). Data are from two independent experiments, each carried out in triplicate. h, Model for the release of up to two molecules of formaldehyde by 5,10-me-THF. All data represent mean ± s.e.m. Statistical significance was assessed using two-tailed Student’s t-tests. *P ≤ 0.05; NS, not significant.
Extended Data Figure 6 Differential toxicity of folate derivatives in HAP1 cells.
Sensitivity of HAP1 wild-type and ∆ADH5 strains to different folate derivatives. Top graphs show those derivatives that are prone to oxidative degradation. Bottom graphs show those derivatives that are resistant. Data are from three independent experiments, each done in triplicate, and represent mean ± s.e.m.
Extended Data Figure 7 Growth analysis of 1C-cycle-deficient HAP1 strains.
a, Glutathione (GSH) levels of all the HAP1 strains used in this study, measured by liquid chromatography–mass spectrometry. Data are from two independent experiments, each performed in triplicate. b, Crystal-violet-stained plates of 1C-cycle-knockout strains proficient (WT) or deficient (∆ADH5) in ADH5, grown in media with (+) or without (–) HT. For ∆MFT;∆SHMT1 strains grown in medium without HT, 4 × 106 cells were plated and grown for 6 days. For the other strains and conditions, 4 × 105 cells were plated and grown until WT was close to confluence. c, Growth curves of HAP1 1C-cycle-defective strains proficient for ADH5, in medium without HT (left) and with HT (right). d, Growth curves of those HAP1 strains without growth defects. Data in c and d are from two independent experiments (each with six replicates). All data represent mean ± s.e.m.
Extended Data Figure 8 ADH5 complementation of 1C-cycle-deficient HAP1 strains.
a–c, Top, western blots showing the expression of ADH5 in ∆ADH5;∆SHMT2 (a), ∆ADH5;∆MFT (b) and ∆ADH5;∆MFT;∆SHMT1 (c) cells complemented with Flag-tagged ADH5. Uncropped scans can be seen in Supplementary Fig. 1. Bottom, cellular sensitivity assays showing that resistance to formaldehyde is restored in the ADH5-complemented strains. Data are from two independent experiments carried out in triplicate. At least two independent clones were used for all the ADH5-complemented strains. d–f, Growth curves in medium with HT of HAP1 1C-cycle-defective strains proficient, deficient (∆ADH5) or complemented (∆ADH5 + ADH5–Flag) for ADH5 expression. Data are from at least two independent experiments, each with six replicates. At least two independent clones were used for all the ADH5-complemented strains. All data represent mean ± s.e.m.
Extended Data Figure 9 THF-derived formaldehyde promotes cell growth of 1C-cycle-deficient cells.
a, Growth of 1C-cycle-deficient HAP1 strains in medium with HT with and without formaldehyde. b, Growth of HAP1 wild-type and ∆ADH5 (without HT, left); ∆MFT and ∆ADH5;∆MFT (with HT, right) cells with a dose range of formaldehyde. c, Growth of HAP1 ∆MFT;∆SHMT1 and ∆ADH5;∆MFT;∆SHMT1 cells in medium without HT with a dose range of formaldehyde. d, Growth of HAP1 ∆SHMT1 and ∆ADH5;∆SHMT1 strains in medium without HT (left); ∆MFT;∆SHMT1 and ∆ADH5;∆MFT;∆SHMT1 strains in medium with HT (right), in the presence of a dose range of formaldehyde. e, Growth of ∆MFT and ∆ADH5;∆MFT HAP1 strains in medium without HT supplemented with a dose range of THF. f, Crystal-violet-stained plates of HAP1 ∆MFT and ∆ADH5;∆MFT strains in medium without HT untreated or treated with THF or formate. g, Growth of HAP1 wild-type and ∆ADH5 (without HT, left); ∆MFT and ∆ADH5;∆MFT (with HT, right) cells with a dose range of THF. h, Growth of HAP1 ∆MFT cells in medium without HT supplemented with a low dose range of THF. Formaldehyde (panels a–d) and THF (panels e–h) were added in increasing amounts each day. Each indicated dose represents the total cumulative dose of formaldehyde or THF. Data in a–e, g and h are from two independent experiments (carried out in triplicate) and are expressed as percentage of cell growth compared to the same strain grown in medium with HT (untreated). All data represent mean ± s.e.m. Statistical significance was assessed using two-tailed Student’s t-tests. *P ≤ 0.05.
Extended Data Figure 10 Formaldehyde detoxification by ADH5 contributes to the 1C pool.
a–c, Tracing of physiological doses of [13C]formaldehyde (FA) to excreted formate in all the HAP1 strains used in this study (a), other cell lines (b) and primary MEFs (c) cultured in medium without HT for 16 h. Data are from two independent experiments carried out in triplicate. d, Plasma levels of serine, glycine and methionine of wild-type (n = 10) and Adh5–/– (n = 11) mice. The plasma samples tested are the same as those shown in Fig. 4e. All data represent mean ± s.e.m. Statistical significance was assessed using two-tailed Student’s t-tests. NS, not significant.
Extended Data Figure 11 Formaldehyde detoxification supplies 1C units for purine and thymine synthesis.
a, Representative mass spectra of dA in HAP1 mutants exposed to physiological doses of [13C]formaldehyde. b, Incorporation of [13C]formate into dA in the presence of 0 or 40 μM formaldehyde in ∆MFT and ∆ADH5;∆MFT HAP1 cells. c, Scheme outlining the use of 13C-labelled formaldehyde to track its predicted incorporation into thymine. dUMP, deoxyuridine monophosphate; dTMP, deoxythymidine monophosphate. d, Left, mass spectra of DNA thymidine (dT) in HAP1 wild-type and ∆MFT cells in the absence or presence of [13C]formaldehyde. The incorporation of one or two atoms of 13C into dT increases the m/z by 1 (M+1) or 2 (M+2) units, respectively. Right, quantitation of left panel. e, Percentage of 13C-labelled dT in ∆MFT and ∆ADH5;∆MFT HAP1 cells untreated or treated with physiological doses of [13C]formaldehyde. f, Incorporation of [13C]formaldehyde into dT in HAP1 wild-type and ∆ADH5 cells. 13C-labelled dA and dT fractions were not corrected for natural abundance (n.a.), which is indicated with a dotted line. [13C]formaldehyde (panels a, d, e and f), [13C]formate and formaldehyde (panel b) were added in increasing amounts each day. Each indicated dose represents the total cumulative dose of the respective compound. Data in b, d, e and f are from three independent experiments. g-i, Tracing of physiological doses of [13C]formaldehyde (FA) to ATP in all the HAP1 strains used in this study (g), other cell lines (h) and primary MEFs (i) cultured in medium without HT for 16 h. Data are from two independent experiments, each carried out in triplicate. 13C-labelled ATP fractions were corrected for natural abundance and are relative to the ATP synthesized de novo during the tracing. dA, dT and ATP were measured by liquid chromatography–mass spectrometry. All data represent mean ± s.e.m. Statistical significance was assessed using two-tailed Student’s t-tests. *P ≤ 0.05; ****P ≤ 0.0001; NS, not significant.
Supplementary information
Supplementary Information
This file contains supplementary figures 1-2 and tables 1-3. (PDF 1638 kb)
Rights and permissions
About this article
Cite this article
Burgos-Barragan, G., Wit, N., Meiser, J. et al. Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature 548, 549–554 (2017). https://doi.org/10.1038/nature23481
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23481
This article is cited by
-
A parallel glycolysis provides a selective advantage through rapid growth acceleration
Nature Chemical Biology (2024)
-
Friend or foe-maldehyde
Nature Chemical Biology (2024)
-
Folic acid alleviated oxidative stress-induced telomere attrition and inhibited apoptosis of neurocytes in old rats
European Journal of Nutrition (2024)
-
Identification of alternative splicing events related to fatty liver formation in duck using full-length transcripts
BMC Genomics (2023)
-
Quantitative detection of formaldehyde using solid phase microextraction gas chromatography–mass spectrometry coupled to cysteamine scavenging
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.