Abstract
Group 2 innate lymphoid cells (ILC2s) regulate inflammation, tissue repair and metabolic homeostasis1, and are activated by host-derived cytokines and alarmins1. Discrete subsets of immune cells integrate nervous system cues2,3,4, but it remains unclear whether neuron-derived signals control ILC2s. Here we show that neuromedin U (NMU) in mice is a fast and potent regulator of type 2 innate immunity in the context of a functional neuron–ILC2 unit. We found that ILC2s selectively express neuromedin U receptor 1 (Nmur1), and mucosal neurons express NMU. Cell-autonomous activation of ILC2s with NMU resulted in immediate and strong NMUR1-dependent production of innate inflammatory and tissue repair cytokines. NMU controls ILC2s downstream of extracellular signal-regulated kinase and calcium-influx-dependent activation of both calcineurin and nuclear factor of activated T cells (NFAT). NMU treatment in vivo resulted in immediate protective type 2 responses. Accordingly, ILC2-autonomous ablation of Nmur1 led to impaired type 2 responses and poor control of worm infection. Notably, mucosal neurons were found adjacent to ILC2s, and these neurons directly sensed worm products and alarmins to induce NMU and to control innate type 2 cytokines. Our work reveals that neuron–ILC2 cell units confer immediate tissue protection through coordinated neuroimmune sensory responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cording, S., Medvedovic, J., Aychek, T. & Eberl, G. Innate lymphoid cells in defense, immunopathology and immunotherapy. Nat. Immunol. 17, 755–757 (2016)
Veiga-Fernandes, H. & Mucida, D. Neuro-immune interactions at barrier surfaces. Cell 165, 801–811 (2016)
Ibiza, S. et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535, 440–443 (2016)
Veiga-Fernandes, H. & Pachnis, V. Neuro-immune regulation during intestinal development and homeostasis. Nat. Immunol. 18, 116–122 (2017)
van de Pavert, S. A. et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508, 123–127 (2014)
Patel, A. et al. Differential RET signaling pathways drive development of the enteric lymphoid and nervous systems. Sci. Signal. 5, ra55 (2012)
Veiga-Fernandes, H. et al. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature 446, 547–551 (2007)
Fonseca-Pereira, D. et al. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature 514, 98–101 (2014)
Veldhoen, M. & Veiga-Fernandes, H. Feeding immunity: skepticism, delicacies and delights. Nat. Immunol. 16, 215–219 (2015)
Kiss, E. A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011)
Spencer, S. P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014)
Robinette, M. L. et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16, 306–317 (2015)
Augood, S. J., Keast, J. R. & Emson, P. C. Distribution and characterisation of neuromedin U-like immunoreactivity in rat brain and intestine and in guinea pig intestine. Regul. Pept. 20, 281–292 (1988)
Ballesta, J. et al. Occurrence and developmental pattern of neuromedin U-immunoreactive nerves in the gastrointestinal tract and brain of the rat. Neuroscience 25, 797–816 (1988)
Honzawa, M., Sudoh, T., Minamino, N., Kangawa, K. & Matsuo, H. Neuromedin U-like immunoreactivity in rat intestine: regional distribution and immunohistochemical study. Neuropeptides 15, 1–9 (1990)
Martinez, V. G. & O’Driscoll, L. Neuromedin U: a multifunctional neuropeptide with pleiotropic roles. Clin. Chem. 61, 471–482 (2015)
Mulligan, L. M. RET revisited: expanding the oncogenic portfolio. Nat. Rev. Cancer 14, 173–186 (2014)
Hoshi, M., Batourina, E., Mendelsohn, C. & Jain, S. Novel mechanisms of early upper and lower urinary tract patterning regulated by RetY1015 docking tyrosine in mice. Development 139, 2405–2415 (2012)
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011)
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010)
Hermann-Kleiter, N. & Baier, G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 115, 2989–2997 (2010)
Howard, A. D. et al. Identification of receptors for neuromedin U and its role in feeding. Nature 406, 70–74 (2000)
Raddatz, R. et al. Identification and characterization of two neuromedin U receptors differentially expressed in peripheral tissues and the central nervous system. J. Biol. Chem. 275, 32452–32459 (2000)
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006)
Monticelli, L. A. et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat. Immunol. 17, 656–665 (2016)
Monticelli, L. A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12, 1045–1054 (2011)
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015)
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013)
Gabanyi, I. et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016)
Muller, P. A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014)
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992)
Cao, X. et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 2, 223–238 (1995)
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998)
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010)
Townsend, M. J., Fallon, P. G., Matthews, D. J., Jolin, H. E. & McKenzie, A. N. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191, 1069–1076 (2000)
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003)
Gautier, L., Cope, L., Bolstad, B. M. & Irizarry, R. A. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20, 307–315 (2004)
Huber, W. et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121 (2015)
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015)
Bouchery, T. et al. ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat. Commun. 6, 6970 (2015)
Joseph, N. M. et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest. 121, 3398–3411 (2011)
Doherty, T. A. et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol. 132, 205–213 (2013)
Zhu, J. D. Myeloid cell-lineage and premylocytic-stage-specific expression of themouse myeloperoxidase gene is controlled at initiation as well as elongation levels of transcription. Cell Res. 9, 107–134 (1999)
Acknowledgements
We thank the Histology and Bioimaging services at iMM Lisboa. We thank the Vivarium and Flow Cytometry platforms at iMM Lisboa and at the Champalimaud Centre for the Unknown. We thank A. McKenzie for providing Il1rl1−/− and Il17rb−/− mice; D. Fonseca-Pereira, V. Fonseca, S. Xapelli and L. Lopes for helpful discussions; and M. Rendas for technical assistance. V.C was supported by Fundação para a Ciência e Tecnologia (FCT), Portugal. J.C. by Fondation pour la Recherche Médicale (FRM), France, and by Marie Skłodowska-Curie fellowship (750030), EU; B.G.-C. by FP7 (289720), EU. N.L.B.-M. is supported by FCT, Portugal, and European Molecular Biology Organisation (EMBO). N.H. by Swiss National Science Foundation (310030_156517). H.V.-F. by ERC (647274), EU; Kenneth Rainin Foundation, USA; Crohn’s and Colitis Foundation of America, USA; and FCT, Portugal.
Author information
Authors and Affiliations
Contributions
V.C. and J.C designed, performed and analysed the experiments in Figs 1, 2, 3, 4 and Extended Data Figs 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. H.R. provided technical assistance in Fig. 4a and managed the animal colony. B. G.-C. contributed to experiments in Figs 1f, g, 3e, f and Extended Data Fig. 1f–g. T.C. analysed the experiments in Fig. 4c, g and Extended Data Figs 7e, f, 8b, c. N.L.B.-M. analysed the experiments in Fig. 1a, b and Extended Data Fig. 1a, b. T.B., K.S. and N.H. contributed to the design of the experiments in Fig. 4, Extended Data Fig. 4c and provided N. brasiliensis larvae and NES. H.V.-F. supervised the work, planned the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks R. Maizels and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
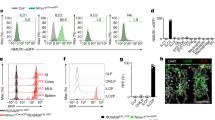
Extended Data Figure 1 Genome-wide ILC2 transcriptional profiling and neuron-ILC2 interactions.
a, Weighted Unifrac PCoA analysis of ILC2s, TH cells, ILC1s and ILC3s. b, Levels of Nmur1 expression in ILC2s, TH cells, ILC1 and ILC3 populations. c, Nmur1 expression in lung ILC2s, eosinophils (Eo), mast cells (Mast), macrophages (Mø), neutrophils (Neu), dendritic cells (DC), helper T (TH), B cells (B) and glial cells (G) (n = 3). d, Nmur2 expression in intestinal ILC2s, eosinophils, mast cells, macrophages, neutrophils, dendritic cells, helper T, B cells, glial cells, neurons and brain (n = 3). e, Nmu expression in lung immune cell subsets (n = 3). f, Distance of T cells and ILC2 to adjacent enteric neurons. T cells (n = 22), ILC2 (n = 28). g, Confocal analysis of lung. Red, neurons (TUBB3); green, Thy1.2; blue, DAPI. Green arrows, candidate ILC2s; red arrow, neuron. Scale bar, 5 μm. h, Confocal analysis of lung. Red, neurons (Chat-Cre-Rosa26RFP); green, CD45.1; blue, DAPI. Green arrows, candidate ILC2s; red arrow, neuron. Scale bar, 5 μm. Error bars show s.e.m. *P < 0.05.
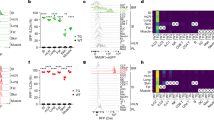
Extended Data Figure 2 Neuromedin U is a potent regulator of innate type 2 cytokines, via NMUR1 activation.
a, ILC2 and TH2 cells were activated with NmU23. ILC2s (n = 6), TH2 cells (n = 3). b, Proliferation (as measured by Ki67 expression) of gut ILC2s upon NmU23 activation in the presence or absence of IL-2 and IL-7 in vitro (n = 3). c, Percentage of Ki67 expression in enteric ILC2 upon NmU23 administration in vivo (n = 5). d, Dot plots representing Ki67 expression in gut ILC2 upon NmU23 administration in vivo. e, Lung innate type 2 cytokines after NmU23 in vitro stimulation (n = 3). f, Dot plots representing lung ILC2-derived type 2 cytokines after NmU23 in vitro activation. g, Enteric ILC2-derived type 2 cytokines upon NmU23 stimulation over different incubation times or PMA+ionomycin (P+I) activation for 4 h. h, Dot plots representing gut ILC2-derived type 2 cytokines upon NmU23 stimulation over different incubation time periods. Error bars show s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
Extended Data Figure 3 NMU is a fast and potent inducer of ILC2 cytokines.
a, Dot plots representing enteric ILC2-derived type 2 cytokines upon activation with increasing concentrations (10, 50 and 100 ng ml−1) of IL-33, IL-25 and NmU23 for 20 h. b, Gut ILC2-derived cytokines after stimulation with increasing concentrations (10, 50 and 100 ng ml−1) of IL-33, IL-25, NmU23 and PMA+ionomycin (P+I) for 4 h (n = 3). c, Lung ILC2- and TH-cell-derived type 2 cytokines after in vivo administration of NmU23 (n = 3). Error bars show s.e.m. *P < 0.05; **P < 0.01; NS, not significant.
Extended Data Figure 4 Activation of ILC2s by NMU and IL-25/IL-33 signals.
a, Lung ILC2s from Il1rl1−/−Il17rb−/− (DKO) and their wild-type controls after NmU23 stimulation (n = 6). b, Type 2 cytokines in Nmur1 sufficient and deficient ILC2s after IL-33 and IL-25 (10 ng ml−1) activation for 24 h (n = 3). c, Intestinal ILC2-derived cytokines after NmU23 administration in Il1rl1−/−Il17rb−/− (DKO) and their wild-type controls. Left panel represents ILC2 percentage gated in total live cells (n = 5). d, Lung ILC2-derived cytokines in wild-type BALB/c and Il1rl1−/−Il17rb−/− bone marrow chimaeras upon NmU23 administration (n = 5). Error bars show s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
Extended Data Figure 5 ILC2-autonomous NMUR1 signals.
a–d, ILC2-derived type 2 cytokines in Nmur1−/− and in their Nmur1+/+ wild-type littermate controls. a, Percentage of intestinal ILC2s and their signature cytokines. Nmur1+/+ (n = 6), Nmur1−/− (n = 9). b, Number of intestinal ILC2s and their signature cytokines. Nmur1+/+ (n = 6); Nmur1−/− (n = 9). c, Percentage of lung ILC2s and their signature cytokines. Nmur1+/+ (n = 6), Nmur1−/− (n = 9). d, Number of lung ILC2s and their signature cytokines. Nmur1+/+ (n = 6), Nmur1−/− (n = 9). e–h, Competitive bone marrow chimaeras. 106 cells of each genotype (CD45.2) were injected intravenously in direct competition with a third-party wild-type competitor (CD45.1/CD45.2), in a 1:1 ratio, into non-lethally irradiated (150 rad) NSG mice (CD45.1). e, Percentage and number of donor ILC2s in the intestine. Nmur1+/+ (n = 8), Nmur1−/− (n = 6). f, Percentage and number of donor ILC2s in the lung. Nmur1+/+ (n = 12), Nmur1−/− (n = 13). g, h, Bone marrow mixed chimaeras upon NmU23 administration. g, Percentage of lung TH-cell-expressing type 2 cytokines. Nmur1+/+ (n = 5), Nmur1−/− (n = 4). h, Number of lung TH-cell-expressing type 2 cytokines. Nmur1+/+ (n = 5), Nmur1−/− (n = 4). Error bars show s.e.m. NS, not significant.
Extended Data Figure 6 Calcineurin inhibition during NMU-dependent ILC2 activation.
Intestinal ILC2 activation with NmU23. Il5, Il13 and Csf2 expression in ILC2s cultured with medium (control), NmU23 or NmU23 and calcineurin inhibitor cyclosporine (CsA) (n = 3). Error bars show s.e.m. ***P < 0.001.
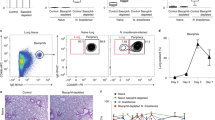
Extended Data Figure 7 Neuromedin U administration during worm infection.
a–f, Mice were infected with N. brasiliensis larvae and treated with NmU23. a, Nmur1 expression in lung ILC2 at day 6 after infection (n = 6). b, Nmur1 expression in lung immune populations (n = 3). c, Number of lung ILC2s at day 1 after infection in NmU23 treated and control animals (n = 5). d, Lung T helper cells at day 1 after infection in NmU23 treated and control animals (n = 5). e, Myeloperoxidase (MPO)- (granulocytes) and Luna-stained (eosinophils) lung sections at day 2 after infection. f, Lung granulocyte and eosinophilic cell counts (cells mm−2) at day 2 after infection (n = 8). Scale bar, 50 μm. Error bars show s.e.m. *P < 0.05; **P < 0.01; NS, not significant.
Extended Data Figure 8 Worm infection in Nmur1 deficient mice.
Nmur1−/− and in their Nmur1+/+ wild-type littermate control mice were infected with N. brasiliensis. a, Number of lung ILC2s and their cytokines at day 6 after infection. Nmur1+/+ (n = 6), Nmur1−/− (n = 8). b, Myeloperoxidase (MPO)- (granulocytes) and Luna-stained (eosinophils) lung sections at day 2 after infection. Scale bar, 50 μm. c, Lung granulocyte and eosinophil cell counts (cells mm−2) at day 2 after infection (n = 8). d, Worm infection burden at day 6 and 9 after infection in the small intestine of Nmur1 sufficient and deficient mice. Day 6 (n = 6), day 9 (n = 5). Error bars show s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
Extended Data Figure 9 Secretory worm products induce ILC2-derived type 2 cytokines.
a, ILC2-derived cytokines after stimulation with Nippostrongylus brasiliensis excretory/secretory products (NES) alone or with NES-activated neurosphere-derived enteric neurons conditioned media (SN NES). Control (n = 3), NES (n = 3), SN NES (n = 3). b, Percentage and number of lung ILC2s and their signature cytokines after intranasal NES administration to wild-type mice. PBS (n = 5), NES (n = 5). Error bars show s.e.m. *P < 0.05; **P < 0.01; NS, not significant.
Extended Data Figure 10 A novel neuron-ILC2 unit orchestrated by Neuromedin U.
Mucosal neurons can directly sense worm products (NES) and the host alarmin (IL-33) to control neuromedin U expression. Neuromedin U activates ILC2s in a cell-autonomous and NMUR1 dependent manner, resulting in a fast and potent production of inflammatory and tissue repair cytokines that confer immediate protection to worm infection. Neuromedin U activates NMUR1 inducing type 2 cytokine expression downstream of ERK phosphorylation and activation of a Ca2+–calcineurin–NFAT cascade. This model indicates that neuron-ILC2 cell units are poised to uniquely ensure potent and immediate type 2 responses in a neuromedin U-dependent manner.
Supplementary information
Rights and permissions
About this article
Cite this article
Cardoso, V., Chesné, J., Ribeiro, H. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017). https://doi.org/10.1038/nature23469
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23469
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.