Abstract
The major energy source for most cells is glucose, from which ATP is generated via glycolysis and/or oxidative metabolism. Glucose deprivation activates AMP-activated protein kinase (AMPK)1, but it is unclear whether this activation occurs solely via changes in AMP or ADP, the classical activators of AMPK2,3,4,5. Here, we describe an AMP/ADP-independent mechanism that triggers AMPK activation by sensing the absence of fructose-1,6-bisphosphate (FBP), with AMPK being progressively activated as extracellular glucose and intracellular FBP decrease. When unoccupied by FBP, aldolases promote the formation of a lysosomal complex containing at least v-ATPase, ragulator, axin, liver kinase B1 (LKB1) and AMPK, which has previously been shown to be required for AMPK activation6,7. Knockdown of aldolases activates AMPK even in cells with abundant glucose, whereas the catalysis-defective D34S aldolase mutant, which still binds FBP, blocks AMPK activation. Cell-free reconstitution assays show that addition of FBP disrupts the association of axin and LKB1 with v-ATPase and ragulator. Importantly, in some cell types AMP/ATP and ADP/ATP ratios remain unchanged during acute glucose starvation, and intact AMP-binding sites on AMPK are not required for AMPK activation. These results establish that aldolase, as well as being a glycolytic enzyme, is a sensor of glucose availability that regulates AMPK.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Salt, I. P., Johnson, G., Ashcroft, S. J. & Hardie, D. G. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem. J. 335, 533–539 (1998)
Ross, F. A., MacKintosh, C. & Hardie, D. G. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 283, 2987–3001 (2016)
Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 45, 31–37 (2017)
Steinberg, G. R. & Kemp, B. E. AMPK in health and disease. Physiol. Rev. 89, 1025–1078 (2009)
Mihaylova, M. M. & Shaw, R. J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023 (2011)
Zhang, C. S. et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 20, 526–540 (2014)
Zhang, Y. L. et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metab. 18, 546–555 (2013)
Oakhill, J. S. et al. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc. Natl Acad. Sci. USA 107, 19237–19241 (2010)
Hawley, S. A. et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 11, 554–565 (2010)
Kroemer, G. & Jäättelä, M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer 5, 886–897 (2005)
Langendorf, C. G. & Kemp, B. E. Choreography of AMPK activation. Cell Res. 25, 5–6 (2015)
Xiao, B. et al. Structural basis of AMPK regulation by small molecule activators. Nat. Commun. 4, 3017 (2013)
Houde, V. P. et al. Investigation of LKB1 Ser431 phosphorylation and Cys433 farnesylation using mouse knockin analysis reveals an unexpected role of prenylation in regulating AMPK activity. Biochem. J. 458, 41–56 (2014)
El-Maghrabi, M. R., Claus, T. H., McGrane, M. M. & Pilkis, S. J. Influence of phosphorylation on the interaction of effectors with rat liver pyruvate kinase. J. Biol. Chem. 257, 233–240 (1982)
Ritterson Lew, C. & Tolan, D. R. Targeting of several glycolytic enzymes using RNA interference reveals aldolase affects cancer cell proliferation through a non-glycolytic mechanism. J. Biol. Chem. 287, 42554–42563 (2012)
Morris, A. J. & Tolan, D. R. Site-directed mutagenesis identifies aspartate 33 as a previously unidentified critical residue in the catalytic mechanism of rabbit aldolase A. J. Biol. Chem. 268, 1095–1100 (1993)
Choi, K. H., Shi, J., Hopkins, C. E., Tolan, D. R. & Allen, K. N. Snapshots of catalysis: the structure of fructose-1,6-(bis)phosphate aldolase covalently bound to the substrate dihydroxyacetone phosphate. Biochemistry 40, 13868–13875 (2001)
Kelley, P. M. & Tolan, D. R. The complete amino acid sequence for the anaerobically induced aldolase from maize derived from cDNA clones. Plant Physiol. 82, 1076–1080 (1986)
Rago, F., Saltzberg, D., Allen, K. N. & Tolan, D. R. Enzyme substrate specificity conferred by distinct conformational pathways. J. Am. Chem. Soc. 137, 13876–13886 (2015)
Forgac, M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929 (2007)
Lu, M., Holliday, L. S., Zhang, L., Dunn, W. A., Jr & Gluck, S. L. Interaction between aldolase and vacuolar H+-ATPase: evidence for direct coupling of glycolysis to the ATP-hydrolyzing proton pump. J. Biol. Chem. 276, 30407–30413 (2001)
Lu, M., Sautin, Y. Y., Holliday, L. S. & Gluck, S. L. The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H+-ATPase. J. Biol. Chem. 279, 8732–8739 (2004)
Lu, M., Ammar, D., Ives, H., Albrecht, F. & Gluck, S. L. Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J. Biol. Chem. 282, 24495–24503 (2007)
Xiao, B. et al. Structure of mammalian AMPK and its regulation by ADP. Nature 472, 230–233 (2011)
Wilson, W. A., Hawley, S. A. & Hardie, D. G. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr. Biol. 6, 1426–1434 (1996)
Li, T. Y. et al. ULK1/2 constitute a bifurcate node controlling glucose metabolic fluxes in addition to autophagy. Mol. Cell 62, 359–370 (2016)
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011)
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protocols 8, 2281–2308 (2013)
Kao, A. W., Noda, Y., Johnson, J. H., Pessin, J. E. & Saltiel, A. R. Aldolase mediates the association of F-actin with the insulin-responsive glucose transporter GLUT4. J. Biol. Chem. 274, 17742–17747 (1999)
Costes, S. V. et al. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 86, 3993–4003 (2004)
Hu, H. et al. Phosphoinositide 3-kinase regulates glycolysis through mobilization of aldolase from the actin cytoskeleton. Cell 164, 433–446 (2016)
Zhao, J. et al. Study of polar metabolites in tobacco from different geographical origins by using capillary electrophoresis–mass spectrometry. Metabolomics 10, (2014)
Zhao, Y. et al. A metabolomics study delineating geographical location-associated primary metabolic changes in the leaves of growing tobacco plants by GC-MS and CE-MS. Sci. Rep. 5, 16346 (2015)
Sols, A. & Marco, R. in Current Topics in Cellular Regulation Vol. 2 (eds Horecker, B. L. & Stadtman, E. R.) 227–273 (Academic, 1970)
Dennis, J. W., Nabi, I. R. & Demetriou, M. Metabolism, cell surface organization, and disease. Cell 139, 1229–1241 (2009)
Acknowledgements
We thank all other members of the S.-C.L. laboratory for suggestions and technical assistance, and the proteomics team at the University of Dundee (D. Lamont, A. Atrih, W. Chen and K. Beattie) for LC–MS analyses of nucleotides. D.G.H. was supported by an Investigator Award from the Wellcome Trust (097726) and a Programme Grant from Cancer Research UK (C37030/A15101). S.-C.L. was supported by grants from the National Key Research and Development Project of China (2016YFA0502001) and the National Natural Science Foundation of China (#31430094, #31690101, #31571214, #31601152 and #J1310027).
Author information
Authors and Affiliations
Contributions
C.-S.Z., S.A.H., Y.Z., M.L., D.G.H. and S.-C.L. conceived the study. C.-S.Z., S.A.H., Y.Z. and M.L. performed most experiments with assistance from T.M., J.C., J.-W.F., A.G., Y.-Q.W., T.Y.L. and S.-Y.L. Y.Z. and Z.W. performed the CE–MS-based analysis of metabolites, and S.A.H., M.L. and M.Z. performed the analysis of adenylates. Z.Y., S.-Y.L., H.Y. and H.-L.P. helped with discussion and interpretation of results. D.G.H. and S.-C.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks D. Tolan and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Glucose deprivation activates AMPK via an AMP/ADP-independent mechanism.
a, Phosphorylation of AMPK (pT172) and ACC (p-ACC) analysed by immunoblotting from the experiment shown in Fig. 1a; the upper panel shows quantification of the pT172:AMPKα ratios (mean ± s.d., n = 2). b, c, Glucose deprivation rapidly activates AMPK without altering cellular AMP/ATP or ADP/ATP ratios in HEK293T cells. Cells were grown in full medium (25 mM glucose, 4 mM alanyl-glutamine, 1 mM pyruvate, 10% serum) and then switched to medium without glucose, with other components unchanged, for 15 min or 2 h. AMPK activation was monitored by immunoblotting for p-AMPKα and p-ACC (b), and intracellular AMP/ATP and ADP/ATP ratios determined by CE–MS (c, results are mean ± s.d., n = 3, N.S., not significant by ANOVA). d, Adenine nucleotide ratios were unaltered in mouse liver after starvation. Mice were fed ad libitum or starved for 16 h, freeze-clamped liver samples prepared, and AMP/ATP and ADP/ATP ratios measured by CE–MS. Results are mean ± s.d., n = 6; ns, not significant by Student’s t-test. e, Decrease in plasma glucose concentrations induced by starving mice for 16 h. Mice from the same experiment shown in Extended Data Fig. 1d were either fed ad libitum or were deprived of food for 16 h and plasma glucose determined. Circles and squares are individual data points and horizontal lines are means ± s.d., n = 6; P value computed by Student’s t-test. f, Extracts were prepared from livers of mice treated as in d (6 mice per group) and analysed by western blotting using the indicated antibodies. g, Western blots from the experiment shown in Fig. 1c. Although shown as separate panels, the blots with each antibody were from the same gel scanned at the same time, and are therefore directly comparable. h, Quantification of blots in g. Results are mean ± s.d., n = 2. i, ADP/ATP ratios determined by LC–MS, from the same experiments shown in Fig. 1c, d. j, Time course of effects of starving MEFs for glucose only, or glucose and glutamine, in the presence of serum. The experiment was as in Fig. 1c except that the medium contained 10% fetal calf serum throughout (n = 2). k, l, ADP/ATP ratios determined by LC–MS, from the experiments shown in Figs 1e–h (n = 3). All experiments were performed at least twice.
Extended Data Figure 2 Glucose deprivation activates AMPK by a mechanism distinct from energy stress.
a, b, d, Axin−/− (a), Lamtor1−/− (b) and CaMKK2 knockdown (d) MEFs were incubated with DMEM containing indicated concentrations of glucose for 2 h. The levels of p-AMPKα and p-ACC in the cell lysates were analysed by immunoblotting. c, AMP/ATP and ADP/ATP ratios in Axin−/− and Lamtor1−/− MEFs, generated as in Fig. 2a, b. Results are mean ± s.d., n = 3. e, Validation of monoclonal anti-β2 antibody for use in immunofluorescence microscopy. Parental wild-type HEK293 cells, or β2 KO cells, were stained with DAPI (blue, nuclei) and anti-β2 antibody (green). Representative merged images are shown, obtained using identical intensity settings. f, Glucose starvation causes translocation of AMPKβ2 to the lysosome in HEK-293 cells that is dependent on N-myristoylation. The experiment was performed in β2 KO cells as in Fig. 1c, except that the lysosomal marker LAMP1 (tagged with RFP) was co-expressed with the wild-type or mutant AMPKβ2. Upper panels show merged images stained blue (4′,6-diamidino-2-phenylindole (DAPI), nuclei), red (LAMP1, lysosomes) and green (AMPKβ2, detected using antibody validated in e), in cells incubated with or without glucose for 20 min. Lower small panels are magnifications of the areas indicated by dashed boxes in the upper panels, showing (L to R) red and green channels and merged images. g, Experiment identical to that shown in Fig. 2c, but with wild-type, G2A or S108A AMPKβ1 expressed in β1 KO cells. Results are mean ± s.d., n = 4. All experiments were performed at least twice.
Extended Data Figure 3 A glycolytic intermediate(s) is responsible for repression of AMPK in the presence of glucose.
All intact cell experiments in this figure were performed in the presence of 10% serum. a, Summary of glycolysis pathway and pathways branching off it. PPP, pentose phosphate pathway; SSP, serine synthesis pathway; HP, hexosamine pathway. b, c, Knockdown of G6PD (glucose-6-phosphate dehydrogenase) (b) or PHGDH (phosphoglycerate dehydrogenase) (c) has no effect on AMPK activation. HEK293T cells were infected with lentivirus expressing siRNA against G6PD (b) or PHGDH (c), or siRNA against GFP as a control; the cells were then incubated in DMEM medium with (25 mM) or without glucose for 2 h, followed by analysis of p-AMPKα and p-ACC levels. d, The hexosamine pathway is not involved in AMPK regulation under glucose deprivation. MEFs were incubated for 2 h in DMEM medium containing GlcNAc (N-acetylglucosamine), a supplement of the hexosamine pathway which cannot be converted back to glucose35, at indicated concentrations and 25 mM or 0 mM glucose. Cells were then subjected to immunoblotting. Experiments in b and c were performed twice, and that in d three times.
Extended Data Figure 4 Absence of FBP defines a starvation signal for AMPK activation.
All intact cell experiments were performed in the presence of 10% serum. a, FBP dissociates axin–LKB1 from LAMTOR1 in vitro. The same experiment identical to that in Fig. 3b was performed except that the bacterially expressed His-tagged AXIN was used in lieu of AXIN-containing cytosol and incubated with light organelles purified from MEFs glucose-starved for 2 h. b, FBP modulates the interaction between AMPK and LKB1. Cytosol from unstarved MEFs was mixed with light organelles purified from 2-h glucose-starved ALDO-TKD MEFs or wild-type MEFs, followed by addition of 200 μM FBP. The mixtures were then dissolved and LKB1 was immunoprecipitated. c, Concentrations of FBP in mouse liver after starvation for 16 h. FBP levels from mouse livers were determined by CE–MS. The amounts of FBP were estimated according to the standard curve generated by plotting the peak-area ratios of unlabelled to 13C-labelled FBP (derived from [U-13C]FBP used as internal standard) against the concentrations of unlabelled FBP. The results were obtained by dividing its amount by the estimated volume of liver, and the concentrations of free-state FBP were then estimated as described previously34. Values are presented as mean ± s.d., n = 5 for each condition, P = 9.627 × 10−5 (Student’s t-test). d, e, Intracellular FBP levels were measured by CE–MS in MEFs after incubation in media containing different concentrations of glucose (d), or in glucose-free DMEM for the indicated time periods (e). Values are presented as mean ± s.d., n = 3 for each treatment; *P = 0.0187, FBP levels in cells incubated in 10 mM glucose (for 1 h) were compared to those in cells incubated with media containing 5 mM glucose (for 1 h); †P = 0.0102, cells incubated with media containing 5 mM to 3 mM glucose (for 1 h); P = 0.831, cells incubated with medium containing 25 mM to 10 mM glucose (for 1 h); P = 0.00577, comparison of incubation in glucose-free medium for 0 min to 20 min (ANOVA). f, Plasma glucose concentrations (left) and intracellular FBP levels (right) in mouse liver. FBP were determined by CE–MS analysis. Results are mean ± s.d., n = 6 for each condition; P value by Student’s t-test. g, h, Linear correlation between extracellular glucose concentrations and intracellular FBP levels. FBP levels in MEFs, measured after 2 h of incubation in media containing various concentrations of glucose as shown in d, and in the starved mouse liver shown in f, were plotted against their corresponding glucose concentrations in the culture medium (g) and plasma (h), respectively. i, FBP levels in SLO-permeabilized MEFs. Levels of FBP in regularly cultured SLO-permeabilized MEFs (left panel), or glucose-starved SLO-permeabilized MEFs treated with exogenous FBP (right panel), were determined by CE–MS analysis. Results are mean ± s.d., n = 3 for each condition. ***P < 0.001, N.S., not significant by Student’s t-test (left panel) and ANOVA (right panel). Experiments shown in left and right panels were performed simultaneously and shared a single control (SLO-permeabilized MEFs incubated in medium containing 25 mM glucose). j–l, Addition of exogenous FBP to permeabilized MEFs blocks glucose starvation-induced AMPK activation. Various glycolytic intermediates (200 μM) as indicated were individually added to the 2-h glucose-starved wild-type (j), PFK1-KD (k) or TPI-KD (l) MEFs pre-incubated with SLO for 5 min. After incubating for another 15 min, cells were lysed and were analysed by immunoblotting. Experiments in this figure were performed twice.
Extended Data Figure 5 Modulation of FBP controls AMPK activation.
All intact cell experiments in this figure were performed in the presence of 10% serum. a, Knockdown of PFK1 activates AMPK in unstarved cells. HEK293T cells were infected with lentivirus expressing PFKP, PFKL and PFKM siRNA, or GFP siRNA as a control, and were incubated in DMEM with or without glucose for 2 h, followed by analysis of p-AMPKα and p-ACC levels. b, c, Effects of overexpression of FBP1 and PFKP on AMPK activation. HEK293T cells were infected with lentivirus expressing FBP1 (b) or PFKP (c), and were then incubated in DMEM with indicated glucose concentrations for 2 h, followed by analysis of p-AMPKα and p-ACC levels. d, Knockdown of Gapdh has no effect on AMPK activation. MEFs were infected with lentivirus expressing Gapdh siRNA, or Gfp siRNA as a control, and were glucose-starved for 2 h, followed by analysis of p-AMPKα and p-ACC levels. Experiments in this figure were performed twice.
Extended Data Figure 6 Knockdown of aldolase renders AMPK activation constitutive.
All intact cell experiments were performed in the presence of 10% serum. a, Schematic diagram showing the strategy for knocking down the three aldolases (isozymes ALDOA–C) in MEFs. In detail, MEFs carrying doxycycline-inducible expression of ALDOA–C were cultured in medium containing doxycycline (Dox, 100 ng/ml), and were infected with lentivirus expressing siRNA against Aldoa, Aldob and Aldoc sequentially, or Gfp siRNA as a control, followed by incubation in doxycycline-free medium for another 12 h. For Fig. 4a, b, #1 siRNAs targeting each enzyme were used. b, Validation of the efficiency and specificity of siRNAs targeting Aldoa–c in MEFs. Cell lysates from MEFs expressing siRNAs against Aldoa, Aldob, Aldoc or Gfp as a control (minus) were immunoblotted as indicated. c, Validation of aldolase-knockdown efficiency by measuring the activity of aldolase in ALDO-TKD cells. Aldolase activities in cell lysates prepared from ALDO-TKD and wild-type MEFs were measured. Results are mean ± s.d., n = 3; P value by ANOVA. d, Knockdown of the three aldolase isozymes renders AMPK activation constitutive. In an experiment identical to Fig. 4a for knocking down the aldolases, the #2 siRNAs targeting each isozyme were used. e, Pyruvate kinase (PK) is not involved in regulation of AMPK. HEK293T cells were infected with lentivirus expressing PKM and PKLR siRNA, or GFP siRNA as a control; the cells were then incubated in DMEM with or without glucose for 2 h, followed by analysis of p-AMPKα and p-ACC levels. f, Knockdown of aldolase does not alter the energy status in MEFs. AMP/ATP and ADP/ATP ratios in wild-type and ALTO-TKD MEFs were measured by CE–MS. Results are mean ± s.d., n = 3; N.S., not significant by Student’s t-test. g–i, Aldolase regulates AMPK through the lysosomal pathway. Aldolases in Lkb1−/− (g), Lamtor1−/− (h) and Camkk2−/− (i) MEFs were knocked down following the procedures described in a, and were incubated with DMEM with 25 mM glucose. The levels of p-AMPKα and p-ACC in the cell lysates were analysed by immunoblotting. j, Triple knockdown of aldolases promotes the formation of ragulator–axin–LKB1–AMPK complex. Endogenous LAMTOR1 in regularly cultured or glucose-starved ALDO-TKD MEFs was immunoprecipitated, followed by immunoblotting. k, Knockdown of aldolase enhances the interaction between AMPK and LKB1. Experiment identical to that shown in j, but endogenous LKB1 was immunoprecipitated. l, FBP fails to dissociate axin–LKB1 from LAMTOR1 on light organelles purified from starved ALDO-TKD MEFs. Cytosol from unstarved MEFs was mixed with light organelles purified from 2-h glucose-starved ALDO-TKD MEFs or wild-type MEFs as a control, and 200 μM FBP was then added to the mixture. The mixtures were then solubilized and LAMTOR1 was immunoprecipitated. m, Addition of exogenous FBP fails to block glucose starvation-induced AMPK activation in permeabilized ALDO-TKD MEFs. FBP (200 μM) was added to the 2-h glucose-starved ALDO-TKD MEFs or wild-type MEFs that had been pre-incubated with SLO for 5 min. After incubating for another 15 min, cells were subjected to immunoblotting. n, FBP is the unique intermediate that modulates aldolase for sensing the availability of glucose. FBP, fructose-2,6-bisphosphate (F26P), and fructose-1-phosphate (F1P) at 200 μM were individually added to light organelles purified from 2-h glucose-starved MEFs. The mixtures were then solubilized and LAMTOR1 was immunoprecipitated, followed by immunoblotting to determine co-precipitated proteins. Experiments in b, e, g–l were performed three times, and those in c, d, f, m and n twice.
Extended Data Figure 7 Axin is constitutively localized to lysosomes in aldolase-deficient MEFs and HEK293T cells.
All intact cell experiments were performed in the presence of 10% serum. a, b, Immunofluorescent staining in wild-type and ALDO-TKD MEFs (a) and HEK293T cells (b) was performed. Rat anti-LAMP2 antibody and goat anti-axin antibody were used to stain MEFs (a), and mouse anti-LAMP1 antibody and goat anti-axin antibody were used to stain HEK293T cells (b). The antibodies had been validated previously6. c, d, Mander’s overlap coefficients of a and b were graphed in c and d, respectively, as mean ± s.d., n = 61 (siGfp, unstarved), 54 (siGfp, GS), 60 (siAldoa–c, unstarved), 58 (siAldoa–c, GS) (c) and n = 57 (siGfp, unstarved), 48 (siGfp, GS), 52 (siAldoa–c, unstarved), 51 (siAldoa–c, GS) (d) for each group; P value by ANOVA. N.S., not significant. The experiment was performed twice.
Extended Data Figure 8 Effects of aldolase mutants on AMPK activation.
All intact cell experiments were performed in the presence of 10% serum. a, ALDOA-D34S mutant increases intracellular FBP levels under glucose starvation. HEK293T cells stably expressing ALDOA or its D34S mutant were incubated in DMEM with or without glucose for 2 h, followed by measuring FBP levels. Results are mean ± s.d., n = 3; *P < 0.05 by ANOVA. N.S., not significant. b, D34S mutant of ALDOA that still binds FBP in glucose-free medium exhibits reduced AMPK activation. ALDO-TKD MEFs stably expressing ALDOA-D34S, or wild-type ALDOA as control, were incubated in DMEM with or without glucose for 2 h, followed by immunoblotting. c, ALDOA-D34S blunts the formation of ragulator–axin–LKB1–AMPK complex. HEK293T cells stably expressing ALDOA and ALDOA-D34S were lysed and the endogenous LAMTOR1 was immunoprecipitated, followed by immunoblotting. d, Adenovirus-mediated expression of the D34S mutant in mouse liver blocks AMPK activation after starvation. Adenovirus expressing ALDOA-D34S, or wild-type ALDOA as control, was injected into the tail veins of 6-week-old mice. Five days later, mice were starved for 16 h. Phosphorylation of AMPKα and ACC was then determined by immunoblotting. e, K230A mutant of aldolase promotes the formation of ragulator–axin–LKB1–AMPK complex. Endogenous LAMTOR1 in unstarved or glucose-starved wild-type MEFs and ALDOA-K230A MEFs was immunoprecipitated, followed by immunoblotting. f, FBP fails to dissociate AXIN–LKB1 from LAMTOR1 on light organelles purified from starved ALDOA-K230A MEFs. Cytosol from unstarved MEFs was mixed with light organelles purified from 2-h glucose-starved ALDOA-K230A MEFs or wild-type MEFs as a control, and 200 μM FBP was then added to each mixture. The mixtures were then dissolved and LAMTOR1 was immunoprecipitated, followed by immunoblotting. g, Addition of exogenous FBP fails to block glucose starvation-induced AMPK activation in permeabilized ALDO-K230A expressing, TKD-MEFs (ALDOA-K230A MEFs). FBP (200 μM) was added to 2-h glucose-starved ALDOA-K230A MEFs or wild-type MEFs that had been pre-incubated with SLO for 5 min. After incubating for another 15 min, cells were subjected to immunoblotting. Experiments in c and f were performed three times, and those in a, b, d, e and g twice.
Extended Data Figure 9 FBP is predominantly derived from glucose.
All intact cell experiments were performed in the presence of 10% serum. a, Isotopomeric distribution of metabolites derived from d-[U-13C]glucose. 13C atoms are marked as filled circles. Note that FBP can be converted from various substrates besides glucose, but the conversion of glucose to F6P and then to FBP is the only way that yields completely M+6-labelled FBP. b, c, MEFs (b) or HEK293T cells (c) were incubated in DMEM without glucose for 4 h. The medium was then added with 25 mM d-[U-13C]glucose. After 15 min or 4 h of incubation, the labelled FBP levels in cells were measured by CE–MS. The results show that FBP produced after re-addition of glucose to the MEFs was almost entirely M+6-labelled. The experiment was performed twice.
Extended Data Figure 10 Aldolase interacts with the v-ATPase–ragulator complex.
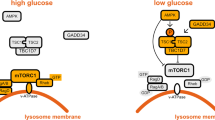
a, A simplified model depicting the idea that aldolase is a sensor of glucose availability that directly links glucose shortage to activation of AMPK. b, HEK293T cells were lysed and the endogenous aldolase was immunoprecipitated, followed by immunoblotting. The experiment was performed twice.
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-3 and the uncropped gels.
Rights and permissions
About this article
Cite this article
Zhang, CS., Hawley, S., Zong, Y. et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548, 112–116 (2017). https://doi.org/10.1038/nature23275
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23275
This article is cited by
-
Ubiquitin ligase subunit FBXO9 inhibits V-ATPase assembly and impedes lung cancer metastasis
Experimental Hematology & Oncology (2024)
-
ACSS2 controls PPARγ activity homeostasis to potentiate adipose-tissue plasticity
Cell Death & Differentiation (2024)
-
Phosphorylation of INF2 by AMPK promotes mitochondrial fission and oncogenic function in endometrial cancer
Cell Death & Disease (2024)
-
Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology
Nature Reviews Molecular Cell Biology (2024)
-
TBC1D23 mediates Golgi-specific LKB1 signaling
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.