Abstract
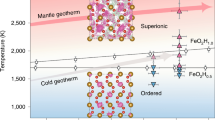
Water transported into Earth’s interior by subduction strongly influences dynamics such as volcanism and plate tectonics1,2,3. Several recent studies have reported hydrous minerals to be stable at pressure and temperature conditions representative of Earth’s deep interior, implying that surface water may be transported as far as the core–mantle boundary4,5,6,7,8. However, the hydrous mineral goethite, α-FeOOH, was recently reported9 to decompose under the conditions of the middle region of the lower mantle to form FeO2 and release H2, suggesting the upward migration of hydrogen and large fluctuations in the oxygen distribution within the Earth system. Here we report the stability of FeOOH phases at the pressure and temperature conditions of the deep lower mantle, based on first-principles calculations and in situ X-ray diffraction experiments. In contrast to previous work suggesting the dehydrogenation of FeOOH into FeO2 in the middle of the lower mantle9, we report the formation of a new FeOOH phase with the pyrite-type framework of FeO6 octahedra, which is much denser than the surrounding mantle and is stable at the conditions of the base of the mantle. Pyrite-type FeOOH may stabilize as a solid solution with other hydrous minerals in deeply subducted slabs, and could form in subducted banded iron formations. Deep-seated pyrite-type FeOOH eventually dissociates into Fe2O3 and releases H2O when subducted slabs are heated at the base of the mantle. This process may cause the incorporation of hydrogen into the outer core by the formation of iron hydride, FeHx, in the reducing environment of the core–mantle boundary.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Inoue, T. Effect of water on melting phase relations and melt composition in the system Mg2SiO4-MgSiO3-H2O up to 15 GPa. Phys. Earth Planet. Inter. 85, 237–263 (1994)
Karato, S., Paterson, M. S. & FitzGerald, J. D. Rheology of synthetic olivine aggregates: influence of grain size and water. J. Geophys. Res. 91, 8151–8176 (1986)
Bercovici, D. & Karato, S. I. Whole-mantle convection and the transition-zone water filter. Nature 425, 39–44 (2003)
Tsuchiya, J. First principles prediction of a new high-pressure phase of dense hydrous magnesium silicates in the lower mantle. Geophys. Res. Lett. 40, 4570–4573 (2013)
Nishi, M. et al. Stability of hydrous silicate at high pressures and water transport to the deep lower mantle. Nat. Geosci. 7, 224–227 (2014)
Ohira, I. et al. Stability of a hydrous δ-phase, AlOOH–MgSiO2(OH)2, and a mechanism for water transport into the base of lower mantle. Earth Planet. Sci. Lett. 401, 12–17 (2014)
Walter, M. J. et al. The stability of hydrous silicates in Earth’s lower mantle: experimental constraints from the systems MgO–SiO2–H2O and MgO–Al2O3–SiO2–H2O. Chem. Geol. 418, 16–29 (2016)
Sano, A. et al. Aluminous hydrous mineral δ-AlOOH as a carrier of hydrogen into the core-mantle boundary. Geophys. Res. Lett. 35, L03303 (2008)
Hu, Q. et al. FeO2 and FeOOH under deep lower-mantle conditions and Earth’s oxygen–hydrogen cycles. Nature 534, 241–244 (2016)
Gleason, A. E., Quiroga, C. E., Suzuki, S., Pentcheva, R. & Mao, W. L. Symmetrization driven spin transition in ε-FeOOH a high pressure. Earth Planet. Sci. Lett. 379, 49–55 (2013)
Nishi, M., Irifune, T., Greaux, S., Tange, Y. & Higo, Y. Phase transitions of serpentine in the lower mantle. Phys. Earth Planet. Inter. 245, 52–58 (2015)
Kuwayama, Y., Hirose, K., Sata, N. & Ohishi, Y. The pyrite-type high-pressure form of silica. Science 309, 923–925 (2005)
Tsuchiya, J. & Tsuchiya, T. First-principles prediction of a high-pressure hydrous phase of AlOOH. Phys. Rev. B 83, 054115 (2011)
Cococcioni, M. & de Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the LDA+U method. Phys. Rev. B 71, 035105 (2005)
Fukai, Y. The iron–water reaction and the evolution of the Earth. Nature 308, 174–175 (1984)
Pépin, C. M., Dewaele, A., Geneste, G., Loubeyre, P. & Mezouar, M. New iron hydrides under high pressure. Phys. Rev. Lett. 113, 265504 (2014)
Bykova, E. et al. Structural complexity of simple Fe2O3 at high pressures and temperatures. Nat. Commun. 7, 10661 (2016)
Hu, Q. et al. Dehydrogenation of goethite in Earth’s deep lower mantle. Proc. Natl Acad. Sci. USA 114, 1498–1501 (2017)
Eberle, M. A., Grasset, O. & Sotin, C. A numerical study of the interaction between the mantle wedge, subducting slab, and overriding plate. Phys. Earth Planet. Inter. 134, 191–202 (2002)
Brown, J. M. & Shankland, T. J. Thermodynamic parameters in the Earth as determined from seismic profiles. Geophys. J. R. Astron. Soc. 66, 579–596 (1981)
Dobson, D. P. & Brodholt, J. P. Subducted banded iron formations as a source of ultralow-velocity zones at the core–mantle boundary. Nature 434, 371–374 (2005)
Wen, L. & Helmberger, D. V. Ultra-low velocity zones near the core-mantle boundary from broadband PKP precursors. Science 279, 1701–1703 (1998)
Richards, M. A., Duncan, R. A. & Courtillot, V. E. Flood basalts and hot-spot tracks: plume heads and tails. Science 246, 103–107 (1989)
Holzapfel, C., Rubie, D., Frost, C. & Langenhorst, F. Fe-Mg interdiffusion in (Mg,Fe)SiO3 perovskite and lower mantle re equilibration. Science 309, 1707–1710 (2005)
Otsuka, K. & Karato, S. Deep penetration of molten iron into the mantle caused by a morphological instability. Nature 492, 243–246 (2012)
Terasaki, H. et al. Stability of Fe-Ni hydride after the reaction between Fe-Ni alloy and hydrous phase (δ-AlOOH) up to 1.2 Mbar: possibility of H contribution to the core density deficit. Phys. Earth Planet. Inter. 194–195, 18–24 (2012)
Nomura, R. et al. Low core-mantle boundary temperature inferred from the solidus of pyrolite. Science 343, 522–525 (2014)
Sano-Furukawa, A. et al. Change in compressibility of δ-AlOOH and δ-AlOOD at high pressure: a study of isotope effect and hydrogen-bond symmetrization. Am. Mineral. 94, 1255–1261 (2009)
Tsuchiya, J. Crystal structure, equation of state, and elasticity of phase H (MgSiO4H2) at Earth’s lower mantle pressures. Sci. Rep. 5, 15534 (2015)
Tsuchiya, T., Tsuchiya, J., Umemoto, K. & Wentzcovitch, R. M. Phase transition in MgSiO3 perovskite in the Earth’s lower mantle. Earth Planet. Sci. Lett. 224, 241–248 (2004)
Dziewonski, A. M. & Anderson, D. L. Preliminary reference Earth model. Phys. Earth Planet. Inter. 25, 297–356 (1981)
Ohishi, Y., Hirao, N., Sata, N., Hirose, K. & Takata, M. Highly intense monochromatic X-ray diffraction facility for high-pressure research at SPring-8. High Press. Res. 28, 163–173 (2008)
Seto, Y., Nishio-Hamane, D., Nagai, T. & Sata, N. Development of a software suite on X-ray diffraction experiments. Rev. High Pressure Sci. Technol. 20, 269–276 (2010)
Tsuchiya, T. First-principles prediction of the P-V-T equation of state of gold and the 660-km discontinuity in Earth’s mantle. J. Geophys. Res. 108, 2462 (2003)
Anderson, O. L., Isaak, D. G. & Yamamoto, S. Anharmonicity and the equation of state for gold. J. Appl. Phys. 65, 1534–1543 (1989)
Yokoo, M., Kawai, K., Nakamura, K., Kondo, Y. & Tsuchiya, T. Ultrahigh-pressure scales for gold and platinum at pressures up to 550 GPa. Phys. Rev. B 80, 104114 (2009)
Dekura, H., Tsuchiya, T., Kuwayama, Y. & Tsuchiya, J. Theoretical and experimental evidence for a new post-cotunnite phase of titanium dioxide with significant optical absorption. Phys. Rev. Lett. 107, 045071 (2011)
Troullier, N. & Martins, J. Efficient pseudopotentials for planewave calculations. Phys. Rev. B 43, 1993–2006 (1991)
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, 7892–7895 (1990)
Wang, X., Tsuchiya, T. & Hase, A. Computational support for a pyrolitic lower mantle containing ferric iron. Nat. Geosci. 8, 556–559 (2015)
Fukui, H., Tsuchiya, T. & Baron, A. Q. R. Lattice dynamics calculations for ferropericlase with internally consistent LDA+U method. J. Geophys. Res. 117, B12202 (2012)
Giannozzi, P. et al. Quantum Espresso: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009)
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976)
Frost, D. J. et al. Experimental evidence for the existence of iron-rich metal in the Earth’s lower mantle. Nature 428, 409–412 (2004)
Ohtani, E., Hirao, N., Kondo, T., Ito, E. & Kikegawa, T. Iron–water reaction at high pressure and temperature, and hydrogen transport into the core. Phys. Chem. Miner. 32, 77–82 (2005)
Tsuchiya, J., Tsuchiya, T., Sano, A. & Ohtani, E. First principles prediction of new high-pressure phase of InOOH. J. Mineral. Petrol. Sci. 103, 116–120 (2008)
Sano, A. et al. X-ray diffraction study of high pressure transition in InOOH. J. Mineral. Petrol. Sci. 103, 152–155 (2008)
Morales, M. A., McMahon, J. M., Pierleoni, C. & Ceperley, D. M. Towards a predictive first-principles description of solid molecular hydrogen with density functional theory. Phys. Rev. B 87, 184107 (2013)
Acknowledgements
We thank Y. Ohishi and N. Hirao for their assistance in the experiments at BL10XU, SPring-8 (proposal numbers 2014B1363 and 2016A1476). We are grateful to T. Irifune for technical supports and discussion. This work was supported by MEXT/JSPS KAKENHI (grant numbers JP15H05469, JP25220712 and JP15H05829 to M.N., JP16H06285 and JP26800274 to Y.K., JP26400516 to J.T., JP26287137 and JP15H05834 to J.T. and T.T.). This research was also supported in part by MEXT as “Exploratory Challenge on Post-K computer” (Frontiers of Basic Science: Challenging the Limits). This research used the computational resources of the K computer provided by the RIKEN Advanced Institute for Computational Science through the HPCI System Research project (Project ID: hp160251/hp170220).
Author information
Authors and Affiliations
Contributions
M.N. and Y.K. carried out the experiments. J.T. and T.T. conducted the first-principles calculations. M.N. and J.T. designed the study and wrote the manuscript. All authors contributed to the discussion of the results and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks F. Guyot and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
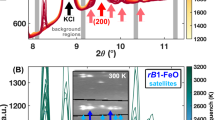
Extended Data Figure 1 XRD patterns of in situ observations.
a, XRD patterns at 94–98 GPa after heating at 2,000 K and >2,400 K. Data were obtained using an X-ray flat-panel detector. Crystallization of CaCl2-type SiO2 from an SiO2 glass pressure medium was recognized at higher temperature. py, pyrite-type FeOOH; FeHx, dhcp-FeHx; SiO2, CaCl2-type SiO2; ppv, Fe2O3 post-perovskite; Au, gold. b, XRD patterns during heating at 100–110 GPa. XRD patterns are shown in order of increasing laser power since the temperature measurements at <1,500 K contain large uncertainties. The temperature was measured to be 1,500 K for a laser power of 35 W using the radiation spectrum. Data were obtained using an X-ray flat-panel detector. XRD peaks corresponding to dhcp-FeHx appeared before the growth of pyrite-type FeOOH. The rapid nucleation rate of Fe compared to FeOOH probably caused the metastable growth of dhcp-FeHx and released oxygen that may have diffused to the pressure medium.
Extended Data Figure 2 FeHx cell volumes (V/Z) derived from XRD patterns as a function of pressure at room temperature.
Filled circles indicate the volumes of dhcp-FeHx obtained in our experiment, which are located between those of hcp-Fe and dhcp-FeH (solid lines)16. Error bars reflect the standard deviations (1σ) derived from various equations of state for gold. The composition was estimated to be FeH0.7 on the basis of volume comparisons.
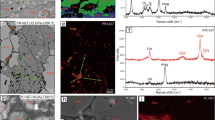
Extended Data Figure 3 Back-scattered electron images of the recovered run products.
a, 40 GPa and 1,223 K. b, 40 GPa and 1,513 K. Two species of CaCl2-type hydroxide with different Fe/Al ratios were produced, as observed in colour contrast. Minerals in dark grey and light grey represent the Al-rich and Fe-rich compositions, respectively. White colour shows an Fe2O3 phase. At higher temperature, the contrast became weak owing to the wider solid-solution range.
Extended Data Figure 4 XRD patterns of the recovered run products.
a, 40 GPa and 1,223 K. b, 40 GPa and 1,513 K. Diffraction peaks from the Au capsule overlap with those from the sample. Red, Fe-rich hydroxide ε-FeOOH; blue, Al-rich hydroxide δ-AlOOH.
Extended Data Figure 5 Cell volumes of solid solution between ε-FeOOH and δ-AlOOH at ambient conditions as a function of the FeOOH component.
A wide solid-solution range was observed in the system FeOOH–AlOOH.
Rights and permissions
About this article
Cite this article
Nishi, M., Kuwayama, Y., Tsuchiya, J. et al. The pyrite-type high-pressure form of FeOOH. Nature 547, 205–208 (2017). https://doi.org/10.1038/nature22823
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22823
This article is cited by
-
Structure and elasticity of CaC2O5 suggests carbonate contribution to the seismic anomalies of Earth’s mantle
Nature Communications (2024)
-
Pressure-induced large volume collapse and possible spin transition in HP-PdF2-type FeCl2
Physics and Chemistry of Minerals (2024)
-
A hydrogen-enriched layer in the topmost outer core sourced from deeply subducted water
Nature Geoscience (2023)
-
Aluminous hydrous magnesium silicate as a lower-mantle hydrogen reservoir: a role as an agent for material transport
Scientific Reports (2022)
-
Superionic iron oxide–hydroxide in Earth’s deep mantle
Nature Geoscience (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.