Abstract
Half of all prostate cancers are caused by the TMPRSS2–ERG gene-fusion, which enables androgens to drive expression of the normally silent E26 transformation-specific (ETS) transcription factor ERG in prostate cells1,2. Recent genomic landscape studies of such cancers3,4,5,6,7,8 have reported recurrent point mutations and focal deletions of another ETS member, the ETS2 repressor factor ERF9. Here we show these ERF mutations cause decreased protein stability and mostly occur in tumours without ERG upregulation. ERF loss recapitulates the morphological and phenotypic features of ERG gain in normal mouse prostate cells, including expansion of the androgen receptor transcriptional repertoire, and ERF has tumour suppressor activity in the same genetic background of Pten loss that yields oncogenic activity by ERG. In the more common scenario of ERG upregulation, chromatin immunoprecipitation followed by sequencing indicates that ERG inhibits the ability of ERF to bind DNA at consensus ETS sites both in normal and in cancerous prostate cells. Consistent with a competition model, ERF overexpression blocks ERG-dependent tumour growth, and ERF loss rescues TMPRSS2–ERG-positive prostate cancer cells from ERG dependency. Collectively, these data provide evidence that the oncogenicity of ERG is mediated, in part, by competition with ERF and they raise the larger question of whether other gain-of-function oncogenic transcription factors might also inactivate endogenous tumour suppressors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005)
Carver, B. S. et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat. Genet. 41, 619–624 (2009)
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015)
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015)
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014)
Kumar, A. et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 22, 369–378 (2016)
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016)
International Cancer Genome Consortium. Prostate cancer - adenocarcinoma. ICGC Cancer Genome Projects https://icgc.org/icgc/cgp/70/508/71331 (2016)
Sgouras, D. N. et al. ERF: an ETS domain protein with strong transcriptional repressor activity, can suppress ets-associated tumorigenesis and is regulated by phosphorylation during cell cycle and mitogenic stimulation. EMBO J. 14, 4781–4793 (1995)
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013)
Twigg, S. R. F. et al. Reduced dosage of ERF causes complex craniosynostosis in humans and mice and links ERK1/2 signaling to regulation of osteogenesis. Nat. Genet. 45, 308–313 (2013)
Hollenhorst, P. C., McIntosh, L. P. & Graves, B. J. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu. Rev. Biochem. 80, 437–471 (2011)
Regan, M. C. et al. Structural and dynamic studies of the transcription factor ERG reveal DNA binding is allosterically autoinhibited. Proc. Natl Acad. Sci. USA 110, 13374–13379 (2013)
Pires, D. E. V., Ascher, D. B. & Blundell, T. L. DUET: a server for predicting effects of mutations on protein stability using an integrated computational approach. Nucleic Acids Res. 42, W314–W319 (2014)
Chen, Y. et al. ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat. Med. 19, 1023–1029 (2013)
Karthaus, W. R. et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175 (2014)
Klezovitch, O. et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc. Natl Acad. Sci. USA 105, 2105–2110 (2008)
Smith, B. A. et al. A basal stem cell signature identifies aggressive prostate cancer phenotypes. Proc. Natl Acad. Sci. USA 112, E6544–E6552 (2015)
Hieronymus, H. et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell 10, 321–330 (2006)
Nelson, P. S. et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc. Natl Acad. Sci. USA 99, 11890–11895 (2002)
Pomerantz, M. M. et al. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat. Genet. 47, 1346–1351 (2015)
Wang, J. et al. Pleiotropic biological activities of alternatively spliced TMPRSS2/ERG fusion gene transcripts. Cancer Res. 68, 8516–8524 (2008)
Balbas, M. D. et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife 2, e00499 (2013)
Fellmann, C. et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Reports 5, 1704–1713 (2013)
Mounir, Z. et al. TMPRSS2:ERG blocks neuroendocrine and luminal cell differentiation to maintain prostate cancer proliferation. Oncogene 34, 3815–3825 (2015)
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR–Cas9 system. Science 343, 80–84 (2014)
Wang, Q., Carroll, J. S. & Brown, M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol. Cell 19, 631–642 (2005)
Feng, J., Liu, T., Qin, B., Zhang, Y. & Liu, X. S. Identifying ChIP–seq enrichment using MACS. Nat. Protoc. 7, 1728–1740 (2012)
Ye, T. et al. seqMINER: an integrated ChIP–seq data interpretation platform. Nucleic Acids Res. 39, e35 (2011)
Arora, V. K. et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155, 1309–1322 (2013)
Rockowitz, S. & Zheng, D. Significant expansion of the REST/NRSF cistrome in human versus mouse embryonic stem cells: potential implications for neural development. Nucleic Acids Res. 43, 5730–5743 (2015)
Machanick, P. & Bailey, T. L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27, 1696–1697 (2011)
Acknowledgements
We thank A. Heguy, H. Hieronymous, J. Li, Y. Liang, E. Peguero, M. Pirun, N. Socci, P. Watson, A. Viale, Y. Zhang, Memorial Sloan Kettering Cancer Center core facilities, and the members of the Sawyers laboratory for comments. R.B. was supported by an American Society of Clinical Oncology (ASCO) Young Investigator Award, a Department of Defense Physician Training Award, and a Prostate Cancer Foundation Young Investigator Award. W.A. was supported by an ASCO Young Investigator Award and Prostate Cancer Foundation Young Investigator Award. M.G.D. was supported by a Howard Hughes Medical Institute (HHMI) Summer Medical Fellowship. N.Sch. is supported by the Prostate Cancer Foundation. C.L.S. is an investigator of the HHMI and this project was supported by National Institutes of Health grants CA155169, CA19387, CA092629, and CA008748.
Author information
Authors and Affiliations
Consortia
Contributions
R.B. and C.L.S. conceived and oversaw the project, performed data interpretation, and co-wrote the manuscript. R.B., E.V.W., W.A., Z.Z., and P.S.S. performed immunoblots, R.B. and P.S.S. performed RNA analysis, W.R.K., P.J.I. and R.B. performed immunohistochemistry, J.W. performed the mouse grafting, R.B. and A.P. prepared experiments for ChIP–seq, and R.B. performed in vitro cell growth assays. W.R.K. made three-dimensional organoid cultures, E.V.W. made bacterially expressed proteins, and R.B., W.R.K., W.A., E.V.W., P.J.I., and M.G.D. cloned plasmid reagents. E.V.W. performed ETS stability analysis, J.A., N.Sch., and R.B. performed analysis of human prostate cancer cohorts, N.Sh. performed gene set enrichment analysis (GSEA), and P.W., Y.Z., and D.Z. performed ChIP–seq analysis. All individual authors made intellectual contributions and reviewed the manuscript. The International SU2C/PCF Cancer Dream provided four unpublished ERF point mutations. The International SU2C-PCF Dream Team is led by A.M.C. and C.L.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks J. Carroll, T. Sato, L. Trotman and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Recurrent ERF loss-of-function mutations are found in prostate cancer and are destabilizing.
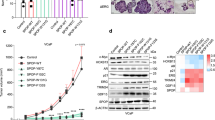
Related to Fig. 1. a, Description of the prostate cancer patient cohorts3,4,5,6,7,8. b, ERG ETS domain (Protein Data Bank accession number 4IRI) illustrating sequence conservation with ERF. ERF missense mutations indicated by sticks. c, Expression of ERF mutant proteins in LNCaP cells. For gel source data, see Supplementary Fig. 1. d, ERF mutant protein densitometry from immunoblot in Extended Data Fig. 1c compared with mRNA RT–qPCR. e, ERF immunoprecipitation in VCaP and MSK-PCa3 cells. f, Left, bacterially expressed ETS domain (hERF20–112) with ERF tumour mutations before (−) or after (+) Ulp1 protease cleavage. Right, densitometry of in vitro ETS domains.
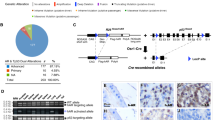
Extended Data Figure 2 Recurrent ERF loss-of-function mutations and focal deletions are found in prostate cancer and are mostly exclusive to tumours without TMPRSS2–ERG.
Related to Fig. 1. a, ERF expression in TCGA-333 cohort4 (n = 333 patients) segregated by copy number loss. Data are Tukey box-and-whisker plots; P value calculated by two-tailed t-test of log2(RSEM values). b, cBio Oncoprint of the SU2C-294 (n = 294) metastatic prostate cancer cohort3. Unlike Fig. 1c, focal ERF deletions could not be ascertained because of differences between TCGA and SU2C copy number data3,4. P value calculated by Fisher’s exact two-tailed test.
Extended Data Figure 3 ERF is a negative regulator of androgen signalling.
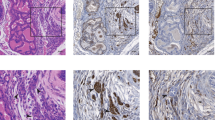
Related to Fig. 2. a, Mouse prostate organoids infected with non-targeting shRNA (shNT) or targeting ERF (shErf_m), grown in three-dimensional culture. For RT–qPCR, n = 2 biological replicates. For gel source data, see Supplementary Fig. 1. b, RT–qPCR analysis of the Pten+/+ organoids; n = 2 biological replicates. c, RNA-seq analysis of organoids derived from Pten+/+ and Pten−/− mouse prostates infected with non-targeting shNT or shErf_m; n = 2 biological replicates. d, Pten+/+ organoid RNA-seq (n = 2 biological replicates) interrogated by GSEA for expression signature of Witte basal prostate cancer18. e, Same data interrogated by GSEA for Nelson androgen up expression signature20.
Extended Data Figure 4 ERF is a negative regulator of androgen signalling.
Related to Fig. 2. a, CWR22Pc cells infected with shRNA targeting human ERF (shERF_1) or a non-targeting shRNA (shNT). For gel source data, see Supplementary Fig. 1. b, Androgen-regulated genes (at least a twofold change, FDR < 0.05 by RNA-seq with 1 nM DHT for 16 h) in CWR22PC cells infected with either shERF_1 or shNT, analysed by the number (left, Venn diagram), the magnitude of expression change (centre, graph), and heat map (right); n = 3 biological replicates. c, RT–qPCR from CWR22Pc cells infected with shERF_1 or shNT. Data are mean ± s.e.m.; n = 3 biological replicates.
Extended Data Figure 5 ERF is a negative regulator of androgen signalling.
Related to Fig. 2. a, Full version of Fig. 2d. Expression profiles of the TCGA-333 primary prostate cancer cohort4 (n = 333 patients) were interrogated for correlation between the ERF mRNA level and two androgen transcriptional activity signatures4,19. P values were calculated by the Spearman correlation test. b, The same analysis as a was applied to the SU2C-150 (n = 150 patients) metastatic castration-resistant prostate cancer cohort3 (mCRPC).
Extended Data Figure 6 ERF and ERG knockdown do not affect androgen receptor levels or its subcellular localization.
Related to Fig. 3. a, VCaP cells infected with doxycycline (dox)-inducible shRNA targeting ERG (+dox, ERG-low; −dox, ERG-high). For gel source data, see Supplementary Fig. 1. b, VCaP cells infected with shRNA targeting ERF (shERF_2) or a non-targeting shRNA (shNT). c, Nuclear/cytoplasmic fractionation of VCaP cells infected with shNT or shERF_2, and the doxycycline-inducible shRNA targeting ERG (shERG). d, RT–qPCR with ERG-low VCaP cells compared with those infected with shERF_2. Data are mean ± s.e.m.; n = 3 biological replicates.
Extended Data Figure 7 ERF and TMPRSS2–ERG have opposing effects on the androgen transcriptome.
Related to Fig. 3. a, Androgen-regulated genes (at least a twofold change, FDR < 0.05 by RNA-seq with 1 nM DHT for 16 h) in VCaP cells infected with a doxycycline-inducible shRNA targeting ERG (with doxycycline, ERG-low; without doxycycline, ERG-high) or a constitutive shRNA targeting ERF (shERF_2) and analysed by number (top) and heat map (bottom left); n = 3 biological replicates. Bottom centre, RNA-seq analysis evaluating the effect of dihydrotestosterone on ERF expression. Bottom right, the effect of doxycycline alone on RNA-seq differential expression analysis. b, RT–qPCR of shERF_2-infected VCaP cells treated ± DHT. Data are mean ± s.e.m.; n = 3 biological replicates. c, Interrogation of RNA-seq in shERF_2 VCaP cells (n = 3 biological replicates) by GSEA for Nelson androgen up expression signature20.
Extended Data Figure 8 ERF and TMPRSS2–ERG have opposing effects on the androgen receptor cistrome.
Related to Fig. 3. a, ChIP–qPCR (n = 2 biological replicates) in VCaP cells, amplifying either the ETS2 promoter region that contains a known ERF binding site9, or an upstream element of PSA27 lacking the ERF binding motif noted in (2) in Fig. 3b. b, The effect of ERF shRNA knockdown on its binding to the ETS2 promoter as assessed by ChIP–qPCR (n = 2 biological replicates), compared with its effect on ERF mRNA by RT–qPCR. Data are mean ± s.e.m.; n = 3 biological replicates. c, ERF ChIP–seq in ERG-high or ERG-low (n = 2 biological replicates: R1, R2) analysed by heat maps. d, Comparison of ERF ChIP–seq peak numbers, n = 2 biological replicates: R1, R2. e, A 10-Mb region illustrating ChIP–seq of ERF binding in ERG-high condition compared with ERG-low. f, ChIP–seq signals for the SCD and PLEKHD1 loci. In both e and f, ChIP–seq signals at the y axis were normalized by read depths.
Extended Data Figure 9 ERG expression decreases the ERF cistrome in normal prostate organoids.
Related to Fig. 3. a, Mouse normal prostate organoids were infected with a TetOn doxycycline-inducible Flag–ERG or empty vector (Flag-alone) and treated with or without doxycycline. For gel source data, see Supplementary Fig. 1. b, ERF ChIP–seq in normal prostate organoids infected with either Flag-alone or Flag–ERG lentivirus, both treated with doxycycline (n = 2 biological replicates: R1, R2), and analysed with heat maps. c, Comparison of ERF ChIP–seq peak numbers, n = 2 biological replicates (R1, R2). d, ERF ChIP–seq signals for the Rph3a1 and Sh3bp5 loci, normalized by read depths.
Extended Data Figure 10 TMPRSS2–ERG activity is mediated, in part, by inactivation of ERF function.
Related to Fig. 4. a, Pooled mouse Pten−/−; R26ERG/ERG organoids infected with CRISPR–Cas9 targeting AAVS1 (sgNT) or ERF (sgErf). For gel source data, see Supplementary Fig. 1. b, Pten−/−; R26ERG/ERG organoids infected instead with a doxycycline-inducible Flag–ERF or empty vector (Flag alone). c, RT–qPCR of mRNA isolated from the Flag–ERF-infected organoids and treated with or without doxycycline, with or without dihydrotestosterone 1 nM for 16 h. Data are mean ± s.e.m.; n = 3 biological replicates. d, Pooled VCaP cells were first infected with non-targeting shRNA (shNT) or ERF (shERF_2), followed by sgNT or sgERF CRISPR–Cas9. e, Androgen-regulated genes (at least a twofold change, FDR < 0.05 by RNA-seq with 1 nM DHT for 16 h) in VCaP cells infected with a doxycycline-inducible shRNA targeting ERG (with doxycycline, ERG-low) and either a constitutive shRNA targeting ERF (shERF_2) or a non-targeting shRNA (shNT). Data analysed by number (left, Venn diagram) and heat map (right); n = 3 biological replicates.
Supplementary information
Supplementary Figures
This file contains a PDF of extended data figures which show gel source data. (PDF 3475 kb)
Source data
Rights and permissions
About this article
Cite this article
Bose, R., Karthaus, W., Armenia, J. et al. ERF mutations reveal a balance of ETS factors controlling prostate oncogenesis. Nature 546, 671–675 (2017). https://doi.org/10.1038/nature22820
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22820
This article is cited by
-
The androgen receptor interacts with GATA3 to transcriptionally regulate a luminal epithelial cell phenotype in breast cancer
Genome Biology (2024)
-
ERG mediates the inhibition of NK cell cytotoxicity through the HLX/STAT4/Perforin signaling pathway, thereby promoting the progression of myocardial infarction
Journal of Physiology and Biochemistry (2024)
-
SND1 binds to ERG and promotes tumor growth in genetic mouse models of prostate cancer
Nature Communications (2023)
-
ETV6 dependency in Ewing sarcoma by antagonism of EWS-FLI1-mediated enhancer activation
Nature Cell Biology (2023)
-
Oncogenic role for an EWS–FLI1 suppressor
Nature Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.