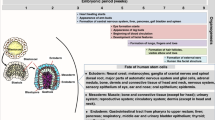
Abstract
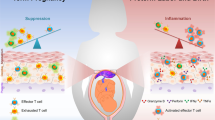
During gestation the developing human fetus is exposed to a diverse range of potentially immune-stimulatory molecules including semi-allogeneic antigens from maternal cells1,2, substances from ingested amniotic fluid3,4, food antigens5, and microbes6. Yet the capacity of the fetal immune system, including antigen-presenting cells, to detect and respond to such stimuli remains unclear. In particular, dendritic cells, which are crucial for effective immunity and tolerance, remain poorly characterized in the developing fetus. Here we show that subsets of antigen-presenting cells can be identified in fetal tissues and are related to adult populations of antigen-presenting cells. Similar to adult dendritic cells, fetal dendritic cells migrate to lymph nodes and respond to toll-like receptor ligation; however, they differ markedly in their response to allogeneic antigens, strongly promoting regulatory T-cell induction and inhibiting T-cell tumour-necrosis factor-α production through arginase-2 activity. Our results reveal a previously unappreciated role of dendritic cells within the developing fetus and indicate that they mediate homeostatic immune-suppressive responses during gestation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
28 June 2017
The name and affiliation for author D.K.H.C. were corrected.
References
Mold, J. E. et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322, 1562–1565 (2008)
Claas, F. H., Gijbels, Y ., van der Velden-de Munck, J. & van Rood, J. J. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science 241, 1815–1817 (1988)
de Vries, J. I., Visser, G. H. & Prechtl, H. F. The emergence of fetal behaviour. II. Quantitative aspects. Early Hum. Dev. 12, 99–120 (1985)
Underwood, M. A., Gilbert, W. M. & Sherman, M. P. Amniotic fluid: not just fetal urine anymore. J. Perinatol. 25, 341–348 (2005)
Campbell, D. E., Boyle, R. J., Thornton, C. A. & Prescott, S. L. Mechanisms of allergic disease—environmental and genetic determinants for the development of allergy. Clin. Exp. Allergy 45, 844–858 (2015)
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra65 (2014)
Haniffa, M. et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 37, 60–73 (2012)
McGovern, N. et al. Human dermal CD14+ cells are a transient population of monocyte-derived macrophages. Immunity 41, 465–477 (2014)
Schuster, C. et al. HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J. Exp. Med. 206, 169–181 (2009)
Schlitzer, A., McGovern, N. & Ginhoux, F. Dendritic cells and monocyte-derived cells: two complementary and integrated functional systems. Semin. Cell Dev. Biol. 41, 9–22 (2015)
Cheng, Y., Wong, M. T., van der Maaten, L. & Newell, E. W. Categorical analysis of human T cell heterogeneity with one-dimensional soli-expression by nonlinear stochastic embedding. J. Immunol. 196, 924–932 (2016)
Guilliams, M. et al. Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45, 669–684 (2016)
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004)
Förster, R., Davalos-Misslitz, A. C. & Rot, A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8, 362–371 (2008)
Wang, X.-N. et al. A three-dimensional atlas of human dermal leukocytes, lymphatics, and blood vessels. J. Investig. Dermatol. 134, 965–974 (2013)
Haynes, B. F. & Heinly, C. S. Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J. Exp. Med. 181, 1445–1458 (1995)
Schuster, C. et al. Development of blood and lymphatic endothelial cells in embryonic and fetal human skin. Am. J. Pathol. 185, 2563–2574 (2015)
Tong, X. Amniotic fluid may act as a transporting pathway for signaling molecules and stem cells during the embryonic development of amniotes. J. Chin. Med. Assoc. 76, 606–610 (2013)
Burlingham, W. J. et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N. Engl. J. Med. 339, 1657–1664 (1998)
Liu, W. et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203, 1701–1711 (2006)
Seddiki, N. et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 (2006)
Elahi, S. et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 504, 158–162 (2013)
Morris, S. M. Jr. Arginine: master and commander in innate immune responses. Sci. Signal. 3, pe27 (2010)
Geiger, R. et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842 (2016)
Ivarsson, M. A. et al. Differentiation and functional regulation of human fetal NK cells. J. Clin. Invest. 123, 3889–3901 (2013)
Polkinghorne, J. Review of the Guidance on the Research Use of Fetuses and Fetal Material (Her Majesty’s Stationery Office, 1989)
Schlitzer, A. et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 (2013)
Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N. & Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284 (2001)
Breitling, R., Armengaud, P., Amtmann, A. & Herzyk, P. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 573, 83–92 (2004)
Acknowledgements
This work was supported by Singapore Immunology Network core funding (F.G. and E.W.N.), Biomedical Research Council (BMRC) Young Investigator Grant (N.McG.), Austrian Science Fund (P19474-B13, W1248-B30 to A.E.-B.), BMRC SPF2014/00 (S.A.), and the Singapore Ministry of Health’s National Medical Research Council (J.K.Y.C, CSIRG/1383/2014, CSA(SI)/008/2016/). We thank L. Robinson for manuscript editing.
Author information
Authors and Affiliations
Contributions
Conceptualization, N.McG., F.G., J.K.Y.C.; methodology, N.McG., F.G., A.S., G.L., D.L., L.J.T., R.M., I.L., N.B.S., H.R.S., E.S., J.L., E.M., S.H., P.S., B.J., C.S., A.E.-B., X.N.W., E.W.N.; clinicians for help accessing samples and for discussion, E.H.K., Y.H.L., M.C., C.N.Z.M., Y.F., T.K.H.L., D.K.H.C., K.-K.T., J.K.C.T., V.B., M.C., M.H., A.S., S.A., A.L., E.W.N.; bioinformatic analysis, N.McG., K.D., M.P., F.G.; writing, N.McG., F.G., J.K.Y.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks V. Soumelis, S. S. Way and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Identification of APC subsets in fetal and adult tissues.
Representative flow plots of gating strategy used to identity APC subsets in fetal and adult tissues. a, Gating strategy used to identify APC populations within the live CD45+, HLA-DR+Lin− gate; CD14+ (red gate), pDC (pink gate), cDC1 (blue gate), and cDC2 (green gate) cells in fetal lung, spleen, skin, and thymus. b, Gating strategy used to identify CD14+ (red gate), pDC (pink gate), cDC1 (blue gate), and cDC2 (green gate) cells in adult lung and spleen. c, Abundance of APC plotted as a percentage of live CD45+ mononuclear cells. Cell abundance was determined in fetal lung and thymus at two time points within the second trimester (12–15 weeks EGA) (circle, lung n = 13, thymus = 9) and 16–22 weeks EGA (square, lung n = 8, thymus n = 8) and compared with adult tissues (triangle, lung n = 8). Mean ± s.e.m. *P < 0.05, ***P < 0.001, Mann–Whitney test. d, Pseudo-colour images of whole-mount fetal spleen (17 weeks EGA) immunolabelled for CD45 (red), CD1c (blue), and CLEC9A (green). White arrows highlight cDC2 (CD45+CD1c+CLEC9A−), white arrowhead highlights cDC1 (CD45+CD1c+CLEC9A+). Scale bar, 5 μm. Representative image of n = 3 experiments shown.
Extended Data Figure 2 Comparison of the transcriptomes and phenotypes of fetal and adult APC subsets.
a, Confirmation of post-sort APC subset purity. Representative dot plots demonstrating cell purity after using FACS to isolate indicated APC subsets from fetal skin and spleen (18–22 weeks EGA), and adult spleen; n = 4. b, Scatter plot of the log(fold change) in gene expression of cDC2 versus cDC1 from fetal and adult skin and spleen. R = 0.92 and P < 2.2 × 10−16. Colours indicate genes upregulated (red) or downregulated (blue) in fetal and adult cDC1 relative to cDC2. c, Scatterplots demonstrating the expression profile of transcription factors important for APC development10, conserved across fetal (blue) and adult (red) spleen. Mean ± s.e.m.
Extended Data Figure 3 Fetal APC populations cluster based on subset after the removal of tissue-specific probes.
a–d, Hierarchical clustering and PCA data before (a, c) and after (b, d) removal of tissue-specific probes. It is clear from the hierarchical clustering (a) that there is strong tissue imprinting in the cells that overwhelms subtype specificity. Upon the removal of tissue-specific probes, cells now cluster based on subtype (b). Also clearly from the PCA plots (c, d), we can see that before tissue gene removal (c), PC1 is entirely determined by tissue. However, upon tissue-specific probe removal (d), PC1 is now devoted to cell type. We identified these tissue-specific genes by finding differentially expressed genes between the pools of all cells from the different tissues (all spleen versus all skin).
Extended Data Figure 4 Fetal and adult spleen cDC have similar phenotypes.
a, b, Characterization of cDC1 (green gate) and cDC2 (cyan gate) across adult and fetal spleen using CyTOF and One-SENSE algorithm11. a, Representative gating strategy used to select input population (red gate) for One-SENSE analysis from fetal (17 weeks EGA) and adult spleen samples. b, Representative data of fetal and adult spleen cDC analysed using the One-SENSE algorithm. The lineage dimension included CD1c and SIRPα as cDC2 markers, and CD26 and CLEC9A as cDC1 markers. The marker dimension included all the other non-lineage markers of the CyTOF panel. Frequency heatmaps of markers expression are displayed for both dimensions. The expression of markers by both adult and fetal spleen cDC1 (green) and cDC2 (cyan) is highlighted with the dashed gates. Representative data from n = 5 donors over two separate experiments.
Extended Data Figure 5 Phenotypic characterization of fetal spleen, thymus, lung, and gut cDC.
a, Representative gating strategy used to select input population (red gate) for One-SENSE analysis from fetal spleen, thymus, lung, and gut (17 weeks EGA). b, Characterization of cDC1 (green gate) and cDC2 (cyan gate) across fetal lung, spleen, thymus, and gut using CyTOF and One-SENSE algorithm11. The lineage dimension included CD1c and SIRPα as cDC2 markers, and CD26 and CLEC9A as cDC1 markers. The marker dimension included all the other non-lineage markers of the CyTOF panel. Frequency heatmaps of markers expression are displayed for both dimensions. The expression of markers by fetal cDC1 (green) and cDC2 (cyan) subsets are highlighted with the dashed gates. Representative data from n = 5 donors over two separate experiments. c, Histograms displaying surface markers differentially expressed across fetal organs (17 weeks EGA) but conserved from fetus to adult. The histograms are generated from CyTOF data (generated as described above). Data are representative of n = 5 donors over two separate experiments. d, Fetal cDC1 (green gate) and cDC2 (blue gate) populations were identified within each organ on the basis of their CD26 and CD1c expression (top panels) by flow cytometry analysis. Using the gates in the top panels to select fetal cDC1 (green contours) and cDC2 (blue contours), intracellular expression of IRF-8 and IRF-4 was determined by flow cytometry. Representative data; n = 3 donors in three experiments.
Extended Data Figure 6 Fetal cDC migrate to lymph nodes.
a, Representative plot of CD14+ cells (red gate), cDC1 (green gate), and cDC2 (blue gate) identified within the MLN-resident (Res) dendritic cell gate (top panels) and migratory (Mig) dendritic cell gate (bottom panels), from a 16 week EGA sample. b, Abundance of cDC1 and cDC2 plotted as a percentage of the total cDC within the resident (left) and migratory (right) fraction within the MLN from 16 to 22 weeks EGA (n = 5). Mean ± s.e.m. c, Histograms comparing the expression of activation markers by resident (pink) and migratory (orange) cDC1 and cDC2 (n = 3). d, RNA from fetal gut and MLN were analysed for the expression of CCL19 and CCL21 from early (13–15 weeks EGA) and late (16–20 weeks EGA) samples (n = 3). Mean ± s.e.m. e, Detection of the proteins CCL19 and CCL21 from lysed fetal gut cells by enzyme-linked immunosorbent assay (n = 3). Mean ± s.e.m. f, Whole-mount immunofluorescence microscopy of 17 week EGA fetal skin from two plains of view. Lymphatic vessels are labelled for LYVE-1 (red), APC are labelled for HLA-DR (green). White arrow indicates APC within lymphatic vessels. Scale bar, 100 μm (left) and 150 μm (right). Representative image of n = 3 experiments shown. g, Gating strategy used to identify CD14+ (red gate), cDC1 (green gate), and cDC2 (blue gate) cells within the supernatant from fetal skin explant left for 48 h in culture and the digested remnant. Representative plots of n = 3 experiments shown.
Extended Data Figure 7 Fetal cDC are sensitive to low concentrations of TLR agonist stimuli.
a, Sort-purified fetal liver and adult spleen cDC2 were cultured with the indicated TLR agonists for 18 h. Cytokines produced were measured in the supernatants by Luminex assay (n = 3). Mean ± s.e.m. b, Heatmap of fetal and adult spleen APC populations of selected genes, including pathogen recognition receptors and co-stimulatory molecules. Heatmap shows the row-based, z-score-normalized gene expression intensities.
Extended Data Figure 8 Fetal cDC promote Treg induction.
a–c, Flow cytometry expression analysis of Treg cells after co-culture for 6 days of adult spleen T cells with fetal (n = 5) or adult (n = 4) spleen cDC2. a, b, Frequency of FOXP3+CD25+ Treg cells (a, red gate) and representative histograms of the intensity of CD127 and CTLA-4 expression by Treg cells (red histograms) and respective isotype controls (grey histograms) are shown (b). c, Composite results showing the frequency of Treg cells plotted as percentage of CD4+ T cells (n ≥ 4). Mean ± s.e.m. d, Bar graph of proliferating CD8+ T cells after 6 days of adult spleen pan T-cell co-culture with fetal (black, n = 4) or adult (grey, n = 4) spleen cDC2. Proliferation was measured by CFSE dilution. Mean ± s.e.m. e, Proliferation of isolated adult spleen CD8+ T cells, after co-culture with fetal spleen cDC2 for 6 days. Left, representative histograms showing CFSE dilution by CD8+ T cells on day 0 (grey histogram) compared with day 6 with (red histogram) or without (black histogram) CD4+ T-cell depletion. Right, cumulative data (n = 4). Mean ± s.e.m. *P < 0.05, **P < 0.01, Mann–Whitney test. f, Fetal spleen cDC1 and cDC2 share immune-suppressive properties. Cytokine detected in co-culture supernatants after T-cell co-culture with fetal cDC1 or cDC2 or adult cDC2 (n = 5). Mean ± s.e.m. Statistical significance represents comparisons between indicated conditions measured by one-way ANOVA, multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; NS, P > 0.05.
Extended Data Figure 9 Gene expression comparison between fetal and adult APC.
a, Heatmap showing the row-based, z-score-normalized gene expression intensities of 3,909 differentially expressed genes between fetal and adult APC. Differentially expressed genes were identified using a t-test with a Benjamini–Hochberg multiple testing corrected P value of <0.05. The genes and cell populations were clustered using the Pearson correlation distance measure and complete linkage method. b, Ingenuity pathway analysis of the differentially expressed genes, >1.5-fold change, between fetal and adult APC. Bars indicate the P values (−log10) for pathway enrichment. Orange squares indicate the ratio of the number of up- or downregulated genes mapped to the enriched pathway, to the total number of molecules on that pathway represented by the dashed orange line. The vertical solid orange line corresponds to the >1.5-fold change threshold. Red arrows highlight pathways involved in DC:T-cell interactions, black arrows highlight pathways associated with iNOS/TNF-α signalling. c, Heatmap of immune-modulatory genes involved in cellular metabolism, immune suppression, and the iNOS/TNF-α signalling. Heatmap shows the row-based, z-score-normalized gene expression intensities. d, e, Microarray (d) and flow cytometry (e) data demonstrating arginase-2 (Arg2) expression by fetal (blue, n = 11) and adult (red, n = 7) APC subsets. Isotype control, grey histogram and square on scatterplot (n = 7). Mean ± s.e.m. f, Fetal and adult cDC2 arginase-2 expression is not mediated by TLR stimulation. Fetal liver and adult spleen cDC2 were sort-purified and stimulated with the indicated TLR agonists or dimethylsulfoxide (DMSO) control for 18 h. cDC2 arginase-2 expression was measured by flow cytometry. Mean ± s.e.m. One-way ANOVA, multiple comparisons test. **P < 0.01. g, TNF-α (n = 4) and Treg (n = 4 and 6) induction after adult spleen T-cell overnight culture alone or with fetal cDC1 or cDC2 for 6 days. Mean ± s.e.m.
Extended Data Figure 10 Fetal cDC regulate T-cell TNF-α production.
a, Ex vivo splenocyte T-cell (bulk tissue cells) and enriched spleen T-cell TNF-α production, representative plots of n = 4. b, Ex vivo co-culture assay where fetal and adult splenocytes were cultured alone or at the indicated ratios of adult:fetal cells (n = 3–4) for 6 days. TNF-α+ and Treg cell induction was determined by flow cytometry analysis. c, d, Scatterplots demonstrating the percentage of TNF-α+ T cells and Treg after the culture of splenocytes under the indicated conditions for 6 days (n ≥ 3). Mean ± s.e.m. Statistical significance represents comparisons between indicated conditions measured by one-way ANOVA, multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; NS, P > 0.05. e–h, Scatterplots demonstrating the percentage (e, f) and absolute cell counts (g, h) of TNF-α+ T cells and Treg after overnight culture of adult spleen T cells alone (n = 6) or co-culture for 6 days with fetal cDC2 in the absence (n = 6) or presence (n = 6) of l-arginine, ABH (n = 4), or BEC (n = 5). Mean ± s.e.m. Statistical significance represents comparisons between indicated conditions measured by one-way ANOVA, multiple comparisons test. *P < 0.05; ***P < 0.001; NS, P > 0.05. i, Fetal dendritic cell arginase activity impacts T-cell TNF-α production but not other pro-inflammatory cytokines. Cytokines detected in co-culture supernatants after adult spleen T-cell co-culture with fetal cDC2 in the absence (n = 5) or presence of l-arginine (1 mM) (n = 5), BEC (30 μM) (n = 3), ABH (30 μM) (n = 5) for 6 days. Mean ± s.e.m. Statistical significance represents comparisons between indicated conditions measured by one-way ANOVA, multiple comparisons test. *P < 0.05; **P < 0.01; NS, P > 0.05. j, Adult spleen T cells were cultured overnight with the indicated of l-arginine, BEC, and ABH (n = 5). Representative flow cytometry plots. k, Cytokines detected in supernatants after adult spleen T cells were cultured alone (in the absence of dendritic cells) for 6 days with or without l-arginine (1 mM), BEC (30 μM), and ABH (30 μM) (n = 5). Mean ± s.e.m. l, m, Fetal spleen cDC (pooled cDC1 and cDC2) and adult spleen cDC2 were cultured alone or in combination at the indicated ratios with adult spleen T cells for 6 days. T-cell TNF-α production (l) and the expansion of Treg cells (m) were assessed by flow cytometry. Statistical significance represents comparisons between indicated conditions measured by one-way ANOVA, multiple comparisons test. *P < 0.05; NS, P > 0.05. Each data point in all the scatter plots represents an individual donor and experiment. Mean ± s.e.m.
Supplementary information
Supplementary Table 1
Conserved Top Ranked genes CD14+ cells. (XLSX 113 kb)
Supplementary Table 2
Conserved Bottom Ranked genes CD14+ cells. (XLSX 84 kb)
Supplementary Table 3
Conserved Top Ranked genes cDC1 cells. (XLSX 106 kb)
Supplementary Table 4
Conserved Bottom Ranked genes cDC1 cells. (XLSX 75 kb)
Supplementary Table 5
Conserved Top Ranked genes cDC2 cells. (XLSX 113 kb)
Supplementary Table 6
Conserved Bottom Ranked genes cDC2 cells. (XLSX 84 kb)
Supplementary Table 7
Ingenuity Pathway Analysis of fetal and adult CD14+ cells conserved genes. (XLSX 40 kb)
Supplementary Table 8
Ingenuity Pathway Analysis of fetal and adult cDC1 conserved genes. (XLSX 41 kb)
Supplementary Table 9
Ingenuity Pathway Analysis of fetal and adult cDC2 conserved genes. (XLSX 39 kb)
Supplementary Table 10
Z scores for list of DEG between fetal and adult APC. (XLSX 448 kb)
Supplementary Table 11
Expression level of DEG between fetal and adult APC. (XLSX 456 kb)
Supplementary Table 12
Ingenuity Pathway Analysis of DEG between fetal and adult APC. (XLSX 21 kb)
Supplementary Table 13
Antibodies used for flow cytometry and CyTOF. (XLSX 53 kb)
Source data
Rights and permissions
About this article
Cite this article
McGovern, N., Shin, A., Low, G. et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 546, 662–666 (2017). https://doi.org/10.1038/nature22795
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22795
This article is cited by
-
The maternal gut microbiome in pregnancy: implications for the developing immune system
Nature Reviews Gastroenterology & Hepatology (2024)
-
Biological Individuality and the Foetus Problem
Erkenntnis (2024)
-
Secrets and lies of host–microbial interactions: MHC restriction and trans-regulation of T cell trafficking conceal the role of microbial agents on the edge between health and multifactorial/complex diseases
Cellular and Molecular Life Sciences (2024)
-
Pregnancy, a test case for immunology
Synthese (2024)
-
Maternal dendritic cells influence fetal allograft response following murine in-utero hematopoietic stem cell transplantation
Stem Cell Research & Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.