Abstract
The lipid bilayer has so far eluded visualization by conventional crystallographic methods, severely limiting our understanding of phospholipid– and protein–phospholipid interactions. Here we describe electron density maps for crystals of Ca2+-ATPase in four different states obtained by X-ray solvent contrast modulation. These maps resolve the entire first layer of phospholipids surrounding the transmembrane helices, although less than half of them are hydrogen-bonded to protein residues. Phospholipids follow the movements of associated residues, causing local distortions and changes in thickness of the bilayer. Unexpectedly, the entire protein tilts during the reaction cycle, governed primarily by a belt of Trp residues, to minimize energy costs accompanying the large perpendicular movements of the transmembrane helices. A class of Arg residues extend their side chains through the cytoplasm to exploit phospholipids as anchors for conformational switching. Thus, phospholipid–Arg/Lys and phospholipid–Trp interactions have distinct functional roles in the dynamics of ion pumps and, presumably, membrane proteins in general.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Toyoshima, C. Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch. Biochem. Biophys. 476, 3–11 (2008)
Toyoshima, C. & Mizutani, T. Crystal structure of the calcium pump with a bound ATP analogue. Nature 430, 529–535 (2004)
Sørensen, T. L., Møller, J. V. & Nissen, P. Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science 304, 1672–1675 (2004)
Toyoshima, C., Norimatsu, Y., Iwasawa, S., Tsuda, T. & Ogawa, H. How processing of aspartylphosphate is coupled to lumenal gating of the ion pathway in the calcium pump. Proc. Natl Acad. Sci. USA 104, 19831–19836 (2007)
Olesen, C. et al. The structural basis of calcium transport by the calcium pump. Nature 450, 1036–1042 (2007)
Toyoshima, C. How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim. Biophys. Acta 1793, 941–946 (2009)
Caffrey, M. & Feigenson, G. W. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry 20, 1949–1961 (1981)
Sonntag, Y. et al. Mutual adaptation of a membrane protein and its lipid bilayer during conformational changes. Nat. Commun. 2, 304 (2011)
Lee, A. G. Biological membranes: the importance of molecular detail. Trends Biochem. Sci. 36, 493–500 (2011)
Gonen, T. et al. Lipid–protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438, 633–638 (2005)
Drachmann, N. D. et al. Comparing crystal structures of Ca2+ -ATPase in the presence of different lipids. FEBS J. 281, 4249–4262 (2014)
Cornelius, F., Habeck, M., Kanai, R., Toyoshima, C. & Karlish, S. J. General and specific lipid-protein interactions in Na,K-ATPase. Biochim. Biophys. Acta 1848, 1729–1743 (2015)
Wood, K. & Zaccai, G. In Biophysical Analysis of Membrane Proteins: Investigating Structure and Function (ed. Pebay-Peyroula, E. ). 213–240 (Wiley-VCH, Weinheim, Germany, 2008)
Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000)
Mouritsen, O. G. & Bloom, M. Mattress model of lipid-protein interactions in membranes. Biophys. J. 46, 141–153 (1984)
Andersen, O. S. & Koeppe, R. E. II . Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36, 107–130 (2007)
Segrest, J. P., De Loof, H., Dohlman, J. G., Brouillette, C. G. & Anantharamaiah, G. M. Amphipathic helix motif: classes and properties. Proteins 8, 103–117 (1990)
Killian, J. A. & von Heijne, G. How proteins adapt to a membrane–water interface. Trends Biochem. Sci. 25, 429–434 (2000)
Rickwood, D., Ford, T. & Graham, J. Nycodenz: a new nonionic iodinated gradient medium. Anal. Biochem. 123, 23–31 (1982)
Toyoshima, C., Nomura, H. & Tsuda, T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432, 361–368 (2004)
Olesen, C., Sørensen, T. L., Nielsen, R. C., Møller, J. V. & Nissen, P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science 306, 2251–2255 (2004)
Toyoshima, C. & Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418, 605–611 (2002)
Picard, M. et al. Ca2+versus Mg2+ coordination at the nucleotide-binding site of the sarcoplasmic reticulum Ca2+-ATPase. J. Mol. Biol. 368, 1–7 (2007)
Brünger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998)
Yamasaki, K., Daiho, T., Danko, S. & Suzuki, H. Multiple and distinct effects of mutations of Tyr122, Glu123, Arg324, and Arg334 involved in interactions between the top part of second and fourth transmembrane helices in sarcoplasmic reticulum Ca2+-ATPase: changes in cytoplasmic domain organization during isometric transition of phosphoenzyme intermediate and subsequent Ca2+ release. J. Biol. Chem. 279, 2202–2210 (2004)
Mares, L. J. et al. Identification of electric-field-dependent steps in the Na+,K+-pump cycle. Biophys. J. 107, 1352–1363 (2014)
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I. & Lomize, A. L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376 (2012)
Silvius, J. R., McMillen, D. A., Saley, N. D., Jost, P. C. & Griffith, O. H. Competition between cholesterol and phosphatidylcholine for the hydrophobic surface of sarcoplasmic reticulum Ca2+-ATPase. Biochemistry 23, 538–547 (1984)
Villamil Giraldo, A. M. et al. Stoichiometry of lipid–protein interaction assessed by hydrophobic photolabeling. FEBS Lett. 580, 607–612 (2006)
Mangialavori, I., Montes, M. R., Rossi, R. C., Fedosova, N. U. & Rossi, J. P. Dynamic lipid–protein stoichiometry on E1 and E2 conformations of the Na+/K+-ATPase. FEBS Lett. 585, 1153–1157 (2011)
Landolt-Marticorena, C., Williams, K. A., Deber, C. M. & Reithmeier, R. A. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol. 229, 602–608 (1993)
Liu, Y. & Nagle, J. F. Diffuse scattering provides material parameters and electron density profiles of biomembranes. Phys. Rev. E 69, 040901 (2004)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–325 (1997)
Lo, V., Kingston, R. L. & Millane, R. P. Determination of molecular envelopes from solvent contrast variation data. Acta Crystallogr. A 65, 312–318 (2009)
Fourme, R., Shepard, W., Kahn, R., L’hermite, G. & Li de La Sierra, I. The Multiwavelength Anomalous Solvent Contrast (MASC) method in macromolecular crystallography. J. Synchrotron Radiat. 2, 36–48 (1995)
Shepard, W., Kahn, R., Ramin, M. & Fourme, R. Low-resolution phase information in multiple-wavelength anomalous solvent contrast variation experiments. Acta Crystallogr. D Biol. Crystallogr. 56, 1288–1303 (2000)
Jiang, J. S. & Brünger, A. T. Protein hydration observed by X-ray diffraction. Solvation properties of penicillopepsin and neuraminidase crystal structures. J. Mol. Biol. 243, 100–115 (1994)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010)
Kraulis, P. J. Molscript: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991)
Wang, B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 115, 90–112 (1985)
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38, 27–28 (1996)
Mori, T., Ishitani, R., Tsukazaki, T., Nureki, O. & Sugita, Y. Molecular mechanisms underlying the early stage of protein translocation through the Sec translocon. Biochemistry 49, 945–950 (2010)
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005)
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010)
MacKerell, A. D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998)
Inesi, G. Sequential mechanism of calcium binding and translocation in sarcoplasmic reticulum adenosine triphosphatase. J. Biol. Chem. 262, 16338–16342 (1987)
Sugita, Y., Ikeguchi, M. & Toyoshima, C. Relationship between Ca2+-affinity and shielding of bulk water in the Ca2+-pump from molecular dynamics simulations. Proc. Natl Acad. Sci. USA 107, 21465–21469 (2010)
Acknowledgements
We thank H. Sakai and H. Okumura of the Japan Synchrotron Radiation Research (JASRI) and members of the Toyoshima laboratory for data collection at BL41XU of SPring-8. Thanks are also due to J. Tsueda, A. Hirata and S. Iwasawa for making crystals for this study. We are grateful to Y. Sugita at RIKEN institute for his help in molecular dynamics simulations and D. B. McIntosh for improving the manuscript. This work is a part of a long-term project (2009B0025, 2012B1486, 2013A0049) at SPring-8 and was supported by a Specially Promoted Project Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan to C.T.
Author information
Authors and Affiliations
Contributions
C.T. planned and supervised the study, carried out the experiments and collected diffraction data. Y.N. wrote the computer programmes, analysed the data and prepared figures. K.H. and N.S. constructed the experimental setup at BL41XU, SPring-8 and wrote device control software. C.T. wrote the manuscript, and all authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks O. Andersen and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Determination of the structure of the lipid bilayer by solvent contrast modulation.
a, The procedure for obtaining the electron density map of a lipid bilayer in crystals of a membrane protein by solvent contrast modulation. The structure of the lipid bilayer is expected to be constant irrespective of the solvent density that can be changed using a contrast medium such as iohexol. The electron density map of the solvent is obtained directly from that of solvent exchange probability. b, Chemical structure of iohexol19(IUPAC name: 5-[N-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodo-N,N′-bis(2,3-dihydroxypropyl)isophthalamide). This compound can be dissolved in water to >80% (w/v) and can increase the electron density of the solvent by 0.10 e− per Å3. c, d, Space-fill models of iohexol according to the chemical formula (c) and after energy minimization (d). e, An X-ray diffraction pattern from an E2(TG) crystal of Ca2+-ATPase soaked in a buffer containing 60% (w/v) iohexol, recorded with an R-Axis V imaging plate detector. Camera length was 600 mm with a He path of 450 mm. The (0, 0, 4) reflection has a Bragg spacing of 146.5 Å. f, A diagram showing the relationship between the observed diffraction intensity and the structure factor from protein plus bilayer and that of solvent. The intensity of each reflection should vary as a quadratic function of solvent electron density  , in which ξ is the concentration of the contrast modulation medium (equation (2)). The diagram shows three different concentrations (ξ = 10, 20, 30%). Solid lines show a diagram for a positive Δθiso (Δθiso+) and dotted lines for a negative Δθiso (Δθiso−). θsolvent represents the phase part of Fsolvent. Refer to equation (2) for the meanings of other symbols. g, Contribution of the lipid bilayer to the structure factor at 0% (black line) and 45% (purple line). Electron density of protein matches with the solvent around 45% iohexol, making the contribution of lipid bilayer more visible. The effect of iohexol is appreciable at Bragg spacing of 10 Å or larger. h, Modification scheme of Pex for phase improvement. Pex is set to 0 for any points within the van der Waals radius from a protein atom and set to 1 for any point in the solvent. Pex is left unmodified for those at dp (distance from the protein surface) < 5.2 Å or those at dm (distance from the bilayer centre) > 40 Å. The inset shows a plot of Pex as a function of the distance from the protein surface when dp was set to 7 Å, showing that Pex = 1 for the points at >5.2 Å away from the surface.
, in which ξ is the concentration of the contrast modulation medium (equation (2)). The diagram shows three different concentrations (ξ = 10, 20, 30%). Solid lines show a diagram for a positive Δθiso (Δθiso+) and dotted lines for a negative Δθiso (Δθiso−). θsolvent represents the phase part of Fsolvent. Refer to equation (2) for the meanings of other symbols. g, Contribution of the lipid bilayer to the structure factor at 0% (black line) and 45% (purple line). Electron density of protein matches with the solvent around 45% iohexol, making the contribution of lipid bilayer more visible. The effect of iohexol is appreciable at Bragg spacing of 10 Å or larger. h, Modification scheme of Pex for phase improvement. Pex is set to 0 for any points within the van der Waals radius from a protein atom and set to 1 for any point in the solvent. Pex is left unmodified for those at dp (distance from the protein surface) < 5.2 Å or those at dm (distance from the bilayer centre) > 40 Å. The inset shows a plot of Pex as a function of the distance from the protein surface when dp was set to 7 Å, showing that Pex = 1 for the points at >5.2 Å away from the surface.
Extended Data Figure 2 Refinement statistics.
a, Squares of structure factors of acentric reflections, |F(−3, 1, l)|2, plotted against solvent electron density. Curves are least squares fits to the experimental data according to equation (2). b, Structure factors of centric reflections, F(−2, 0, l), plotted against solvent electron density. Lines show least squares fits to the experimental data according to equation (3). Error bars in a and b represent  and
and  , respectively. c, Rlsq as defined in equation (5) before and after refining
, respectively. c, Rlsq as defined in equation (5) before and after refining  and scale. d, e, R-factors (as defined in equation (7)) for the diffraction data (200 to 20 Å (d) and 20 to 3.2 Å (e) Bragg spacings) from E1⋅AlF4−⋅ADP⋅2Ca2+ crystals of Ca2+-ATPase. The atomic model used was derived from the crystals soaked in 45% (w/v) iohexol. The number associated with each plot refers to iohexol concentration. f, Rcullis as defined in equation (9), showing a large decrease from the flat model (that is, no lipid bilayer; blue) by modelling the bilayer (red) at Bragg spacing of 25 Å or larger. g, Figure of merit (FOM as defined in equation (8)) for the phases derived from the flat (blue) or refined (red) model All the data in a–g were derived from the E1⋅AlF4−⋅ADP⋅2Ca2+ crystals. h, Phase differences between the flat and the refined models, showing that the phase errors in the flat model may be substantial. Here no weighting scheme according to the amplitude is applied. i, Crystallographic R-factors before (open circles) and after (filled circles) introducing 30 DOPC molecules into the atomic model of Ca2+-ATPase in E2(TG). Incorporation of phospholipid molecules into the atomic models improved the R-factor for the resolution range between 200 to 20 Å from 0.608 to 0.393 for E2(TG), from 0.472 to 0.384 for E1⋅2Ca2+, from 0.622 to 0.383 for E1⋅AlF4−⋅ADP⋅2Ca2+, from 0.590 to 0.377 for the E2⋅AlF4−(TG) crystal of C2 symmetry and from 0.662 to 0.410 for the same crystal of P21212 symmetry. Here the CNS mask refers to that used in CNS version 1.2 (ref. 24), in which the value is 0 for any point within the solvent radius (1.4 Å) from the protein van der Waals surface or 1 otherwise. The ‘soft mask’ refers to a smoothed mask by applying Wang’s method for solvent flattening40(flattening radius of 5 Å).
and scale. d, e, R-factors (as defined in equation (7)) for the diffraction data (200 to 20 Å (d) and 20 to 3.2 Å (e) Bragg spacings) from E1⋅AlF4−⋅ADP⋅2Ca2+ crystals of Ca2+-ATPase. The atomic model used was derived from the crystals soaked in 45% (w/v) iohexol. The number associated with each plot refers to iohexol concentration. f, Rcullis as defined in equation (9), showing a large decrease from the flat model (that is, no lipid bilayer; blue) by modelling the bilayer (red) at Bragg spacing of 25 Å or larger. g, Figure of merit (FOM as defined in equation (8)) for the phases derived from the flat (blue) or refined (red) model All the data in a–g were derived from the E1⋅AlF4−⋅ADP⋅2Ca2+ crystals. h, Phase differences between the flat and the refined models, showing that the phase errors in the flat model may be substantial. Here no weighting scheme according to the amplitude is applied. i, Crystallographic R-factors before (open circles) and after (filled circles) introducing 30 DOPC molecules into the atomic model of Ca2+-ATPase in E2(TG). Incorporation of phospholipid molecules into the atomic models improved the R-factor for the resolution range between 200 to 20 Å from 0.608 to 0.393 for E2(TG), from 0.472 to 0.384 for E1⋅2Ca2+, from 0.622 to 0.383 for E1⋅AlF4−⋅ADP⋅2Ca2+, from 0.590 to 0.377 for the E2⋅AlF4−(TG) crystal of C2 symmetry and from 0.662 to 0.410 for the same crystal of P21212 symmetry. Here the CNS mask refers to that used in CNS version 1.2 (ref. 24), in which the value is 0 for any point within the solvent radius (1.4 Å) from the protein van der Waals surface or 1 otherwise. The ‘soft mask’ refers to a smoothed mask by applying Wang’s method for solvent flattening40(flattening radius of 5 Å).
Extended Data Figure 3 Solvent exchange probability maps for the transmembrane regions of Ca2+-ATPase crystals.
a–d, E1⋅2Ca2+ (a), E1·AlF4−·ADP⋅2Ca2+ (b), E2⋅AlF4−(TG) (c) and E2(TG) (d). Grey mesh represents Pex of 0.3, blue mesh, 0.6 and purple, 0.9, calculated at 4.5 Å resolution. Cyan spheres (I and II, also circled) represent bound Ca2+ and small red spheres crystal water. Cylinders represent α-helices (M1–M10). Trp, Tyr, Lys and Arg residues in the transmembrane region and thapsigargin (TG) are shown in sticks. Stereo pictures, viewed parallel to the membrane. Note that the map shows that iohexol comes very close to the Ca2+-binding sites in E1·2Ca2+ (a), consistent with the observations that site II Ca2+ is exchangeable with Ca2+ in the cytoplasm46, and from the lumenal side, water molecules can reach just below site I Ca2+ (ref. 47).
Extended Data Figure 4 Outer layer phospholipids and putative detergent islands.
a, A map showing the distance of density peak from the bilayer centre in the E1⋅2Ca2+ crystal. Viewed perpendicular to the bilayer. The boxes (solid line for the cytoplasmic leaflet; dotted lines for the luminal leaflet) specify the area shown in b. b, c, Contrast modulation electron density maps for the bilayer region in the E1⋅2Ca2+ (b) and E2(TG) (c) crystals, viewed parallel to the bilayer. Calculated at 4.5 Å resolution for a slab of 5 Å thick (cyan, 0.38 e− per Å3; blue, 0.44 e− per Å3). Partial atomic models of phospholipid (up to the carbonyl group; in ball and stick) are superimposed. Purple numbers identify the first-layer phospholipids in the cytoplasmic leaflet, orange identifies those in the lumenal leaflet and green numbers indicate outer-layer phospholipids. Asterisks represent putative detergent islands (peak-to-peak distance <24 Å). The detergent used here, octaethyleneglycol mono-n-dodecylether (C12E8), is shown with sticks at the right end in b.
Extended Data Figure 5 Identification of phospholipid molecules in the crystals of Ca2+-ATPase.
a–d, E1⋅2Ca2+ (a), E1⋅AlF4−⋅ADP⋅2Ca2+ (b), E2(TG) (c) and E2⋅AlF4−(TG) (C2 symmetry) (d). Atomic models for the head groups appear in sticks with orange balls for phosphorous atoms. Purple labels identify the first-layer phospholipids in the cytoplasmic leaflet, orange labels those in the luminal leaflet and green labels outer-layer lipids. Lipid molecules shared by adjacent Ca2+-ATPase molecules bear two labels. The boxes (solid line for the cytoplasmic leaflet; dotted line for the luminal leaflet) in a and c specify the areas shown in Extended Data Fig. 4b, c. Asterisks represent putative detergent islands (peak-to-peak distance <24 Å). The cylinders represent transmembrane helices (M1–M10). The side chains of Trp, Tyr, Lys and Arg are shown as green sticks. Thin grey lines identify unit cell boundaries. The purple and green boxes in d (solid line for the cytoplasmic leaflet; dotted lines for the luminal leaflet) specify the area shown in Extended Data Fig. 7a, b, respectively. Viewed perpendicular to the bilayer plane. Note that all the phospholipid molecules in the E2(TG) crystal (20 in the cytoplasmic leaflet and 15 in the luminal one) are modelled, but only two are surrounded by phospholipids alone.
Extended Data Figure 6 Effects of crystal packing on the disposition of the first-layer phospholipids.
a–f, Contrast modulation electron density maps for the first layer phospholipids in the E2⋅AlF4−(TG) crystals of Ca2+-ATPase with a C2 (a, c and e) or a P21212 (b, d and f) symmetry. Calculated at 4.5 Å resolution (cyan, 0.38 e−1 per Å3; blue, 0.46 e−1 per Å3). a, b, The cytoplasmic leaflet, viewed perpendicular to the bilayer from the cytoplasm. c, d, Viewed parallel to the bilayer. e, f, The lumenal leaflet viewed perpendicular to the bilayer from the lumenal side. 20 and 17 (C2), and 20 and 16 (P21212) phospholipid molecules are identified in the cytoplasmic and lumenal leaflets, respectively. Partial atomic models of phospholipid (up to the carbonyl group; in ball and stick) are superimposed. The cylinders represent α-helices. Trp, Tyr, Lys and Arg side chains appear in lime sticks and TG in violet sticks. Ca2+-ATPase in the P21212 crystal is aligned to that in the C2 crystal with the M1–M10 helices, resulting in tilting of 11.3° with respect to the ab plane of the C2 crystal.
Extended Data Figure 7 Effects of crystal packing on the disposition of outer-layer phospholipids.
a–d, Contrast modulation electron density maps for the bilayer region distant from the protein surface in the E2⋅AlF42−(TG) crystals of C2 (a, b) and P21212 (c, d) symmetries. Calculated at 4.5 Å resolution (cyan mesh, 0.38 e− per Å3). Viewed in two orthogonal directions parallel to the membrane. The slab is 15 Å thick and specified in Extended Data Fig. 5d. Here the xy plane coincides with the ab plane of the crystal, and the orientation of Ca2+-ATPase is different between the two crystal forms. Purple dotted lines in c and d show the positions of the bilayer centre, which is tilted by 11.3° to the ab plane. The root mean square deviation between the protein Cα atoms in the two crystal forms is 0.81 Å. TG is shown with violet sticks.
Extended Data Figure 8 Evaluation of one-dimensional models for the bilayer.
a, A simple one-dimensional model for the solvent and bilayer parts used in the initial stage (left). Electron density profiles of such models (right). Black lines approximately follow the density profile of DOPC bilayer obtained by small-angle X-ray diffraction32. 80% iohexol (w/v) increases the solvent electron density by 0.1 e− per Å3. b, c, Electron density profiles of four one-dimensional models for the E1⋅AlF4−⋅ADP⋅2Ca2+ crystal before (b) and after (c) refinement. In the initial models (b), the peak-to-peak distance is varied from 30–50 Å or the peak electron density is reduced to 0.40 e− per Å3 for the peak-to-peak distance of 40 Å. d, Effect of the inclination (0° to 16.7° with respect to the ab plane) of the bilayer in the initial one-dimensional model for the E2⋅AlF4−(TG) crystal with a P21212 symmetry. The R-factor for the diffraction data (200 to 12 Å Bragg spacing; equation (7)) after refinement for 0, 50 and 70% of iohexol. At 11.3° of inclination, Trp residues in the cytoplasmic leaflet become aligned with the bilayer plane. Also see Extended Data Fig. 7c, d.
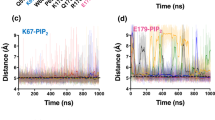
Extended Data Figure 9 All atom molecular dynamics simulations of phospholipids that surround Ca2+-ATPase.
a–d, E1⋅2Ca2+ (a), E1~P⋅ADP⋅2Ca2+ (b), E2~P (c) and E2 (d). Viewed from the cytoplasm (left panels) and the lumen of the sarcoplasmic reticulum (right panels) perpendicular to the membrane. The plus symbol identifies the initial locations of the first-layer phospholipids (phosphorous atoms in the head groups) in the cytoplasmic (left panel with purple numbers) and the lumenal (right panel with orange numbers) leaflets, respectively. The phospholipids marked also with circles indicate that refined atomic models were used and those without indicate that direct modelling was impossible and the initial model was taken from those in other states. Pink and blue dots represent the locations of the phosphorus atoms in the first (pink) and outer (blue) layer of phospholipids during molecular dynamics simulations, plotted for 5 ns (between 95 and 100 ns) at 0.1-ns intervals.
Supplementary information
Supplementary Information
This file contains Supplementary Discussions 1-3, Supplementary Tables 1-8 and Supplementary References. (PDF 427 kb)
Movements of the calcium pump and the first layer phospholipids during the reaction cycle as deduced from the crystal structures
A view from the M3-M7 side, corresponding to the left panels in Fig. 3. Orange spheres represent the phosphorus atoms of the first layer phospholipids at the front of the ATPase and the grey ones those at the back. (MP4 7707 kb)
Hypothetical movements of the calcium pump and the first layer phospholipids arising from the conventional alignment with the M7-M10 transmembrane helices
A view from the M6-M9 side as in the right panels in Fig. 3. Orange spheres represent the phosphorus atoms of the first layer phospholipids at the front of the ATPase and the grey ones those at the back. Note that phospholipid 13 (slate blue) goes far below and 23 (slate blue) goes high above the membrane plane in E1~P•ADP•2Ca2+. (MP4 7530 kb)
Movements of the calcium pump and the first layer phospholipids during the reaction cycle as deduced from the crystal structures
A view from the M3-M7 side, corresponding to the right panels in Fig. 3. Orange spheres represent the phosphorus atoms of the first layer phospholipids at the front of the ATPase and the grey ones those at the back. Note that phospholipids 13 and 23 stay at almost the same level during the reaction cycle. (MP4 7679 kb)
Rights and permissions
About this article
Cite this article
Norimatsu, Y., Hasegawa, K., Shimizu, N. et al. Protein–phospholipid interplay revealed with crystals of a calcium pump. Nature 545, 193–198 (2017). https://doi.org/10.1038/nature22357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22357
This article is cited by
-
Genetically encoded bioorthogonal tryptophan decaging in living cells
Nature Chemistry (2024)
-
Conformational cycle of human polyamine transporter ATP13A2
Nature Communications (2023)
-
Electrostatic switch mechanisms of membrane protein trafficking and regulation
Biophysical Reviews (2023)
-
Electrostatic interactions between single arginine and phospholipids modulate physiological properties of sarcoplasmic reticulum Ca2+-ATPase
Scientific Reports (2022)
-
Gaseous NO2 induces various envelope alterations in Pseudomonas fluorescens MFAF76a
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.