Abstract
Brain enlargement has been observed in children with autism spectrum disorder (ASD), but the timing of this phenomenon, and the relationship between ASD and the appearance of behavioural symptoms, are unknown. Retrospective head circumference and longitudinal brain volume studies of two-year olds followed up at four years of age have provided evidence that increased brain volume may emerge early in development1,2. Studies of infants at high familial risk of autism can provide insight into the early development of autism and have shown that characteristic social deficits in ASD emerge during the latter part of the first and in the second year of life3,4. These observations suggest that prospective brain-imaging studies of infants at high familial risk of ASD might identify early postnatal changes in brain volume that occur before an ASD diagnosis. In this prospective neuroimaging study of 106 infants at high familial risk of ASD and 42 low-risk infants, we show that hyperexpansion of the cortical surface area between 6 and 12 months of age precedes brain volume overgrowth observed between 12 and 24 months in 15 high-risk infants who were diagnosed with autism at 24 months. Brain volume overgrowth was linked to the emergence and severity of autistic social deficits. A deep-learning algorithm that primarily uses surface area information from magnetic resonance imaging of the brain of 6–12-month-old individuals predicted the diagnosis of autism in individual high-risk children at 24 months (with a positive predictive value of 81% and a sensitivity of 88%). These findings demonstrate that early brain changes occur during the period in which autistic behaviours are first emerging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hazlett, H. C. et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch. Gen. Psychiatry 62, 1366–1376 (2005)
Hazlett, H. C. et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry 68, 467–476 (2011)
Ozonoff, S. et al. A prospective study of the emergence of early behavioral signs of autism. J. Am. Acad. Child Adolesc. Psychiatry 49, 256–266.e1, 2 (2010)
Zwaigenbaum, L. et al. Behavioral manifestations of autism in the first year of life. Int. J. Dev. Neurosci. 23, 143–152 (2005)
Piven, J. et al. An MRI study of brain size in autism. Am. J. Psychiatry 152, 1145–1149 (1995)
Courchesne, E. et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57, 245–254 (2001)
Schumann, C. M. et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 30, 4419–4427 (2010)
Sparks, B. F. et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59, 184–192 (2002)
Shen, M. D. et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 136, 2825–2835 (2013)
Lord, C., Rutter, M., DiLavore, P. C. & Risi, S. The Autism Diagnostic Observation Schedule. (Western Psychological Services, 2000)
Wetherby, A. & Prizant, B. Communication and Symbolic Behavior Scales Developmental Profile, First Normed Edition. (Paul H. Brookes, 2002)
Li, G. et al. Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cereb. Cortex 24, 1289–1300 (2014)
Elison, J. T. et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am. J. Psychiatry 170, 899–908 (2013)
Estes, A. et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J. Neurodev. Disord. 7, 24 (2015)
Qureshi, A. Y. et al. Opposing brain differences in 16p11.2 deletion and duplication carriers. J. Neurosci. 34, 11199–11211 (2014)
Bernier, R. et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 158, 263–276 (2014)
Panizzon, M. S. et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex 19, 2728–2735 (2009)
Rakic, P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 18, 383–388 (1995)
Chenn, A. & Walsh, C. A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 (2002)
Casanova, M. F. et al. Minicolumnar abnormalities in autism. Acta Neuropathol. 112, 287–303 (2006)
Hill, J. et al. Similar patterns of cortical expansion during human development and evolution. Proc. Natl Acad. Sci. USA 107, 13135–13140 (2010)
Packer, A. Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci. Biobehav. Rev. 64, 185–195 (2016)
Fang, W. Q. et al. Overproduction of upper-layer neurons in the neocortex leads to autism-like features in mice. Cell Reports 9, 1635–1643 (2014)
Pucilowska, J. et al. The 16p11.2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK pathway. J. Neurosci. 35, 3190–3200 (2015)
Nonaka-Kinoshita, M. et al. Regulation of cerebral cortex size and folding by expansion of basal progenitors. EMBO J. 32, 1817–1828 (2013)
Sugathan, A. et al. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proc. Natl Acad. Sci. USA 111, E4468–E4477 (2014)
Cotney, J. et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat. Commun. 6, 6404 (2015)
Marchetto, M. C. et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2016.95 (2016)
Charman, T. Early identification and intervention in autism spectrum disorders: some progress but not as much as we hoped. Int. J. Speech Lang Pathol. 16, 15–18 (2014)
Landa, R. J., Gross, A. L., Stuart, E. A. & Faherty, A. Developmental trajectories in children with and without autism spectrum disorders: the first 3 years. Child Dev. 84, 429–442 (2013)
Messinger, D. et al. Beyond autism: a baby siblings research consortium study of high-risk children at three years of age. J. Am. Acad. Child Adolesc. Psychiatry 52, 300–308.e1 (2013)
Georgiades, S. et al. A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. JAMA Psychiatry 70, 42–48 (2013)
Ozonoff, S. et al. The broader autism phenotype in infancy: when does it emerge? J. Am. Acad. Child Adolesc. Psychiatry 53, 398–407.e2 (2014)
Hazlett, H. C. et al. Brain volume findings in 6-month-old infants at high familial risk for autism. Am. J. Psychiatry 169, 601–608 (2012)
Lord, C., Rutter, M. & Le Couteur, A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 24, 659–685 (1994)
Mullen, E. M. Mullen Scales of Early Learning: AGS edn (American Guidance Service, 1995)
Sparrow, S., Balla, D. & Cicchetti, D. Vineland Scales of Adaptive Behavior: A Survey Form Manual. (American Guidance Service, 1984)
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th edn) (Washington, 2000)
Guthrie, W., Swineford, L. B., Nottke, C. & Wetherby, A. M. Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. J. Child Psychol. Psychiatry 54, 582–590 (2013)
Wang, J. et al. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front. Neuroinform. 8, 7 (2014)
Shaw, P. et al. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol. Psychiatry 72, 191–197 (2012)
Shaw, P. et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28, 3586–3594 (2008)
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002)
Kim, S. H. et al. Adaptive prior probability and spatial temporal intensity change estimation for segmentation of the one-year-old human brain. J. Neurosci. Methods 212, 43–55 (2013)
Gilmore, J. H. et al. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb. Cortex 22, 2478–2485 (2012)
Chawarska, K. et al. Early generalized overgrowth in boys with autism. Arch. Gen. Psychiatry 68, 1021–1031 (2011)
WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Growth Velocity based on Weight, Length and Head Circumference: Methods and Development. (WHO, 2009)
Im, K. et al. Brain size and cortical structure in the adult human brain. Cereb. Cortex 18, 2181–2191 (2008)
Gilmore, J. H. et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 27, 1255–1260 (2007)
Benjamini, Y., Krieger, A. M. & Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometricka 93, 491–507 (2006)
Hinton, G. E. & Salakhutdinov, R. R. Reducing the dimensionality of data with neural networks. Science 313, 504–507 (2006)
Acknowledgements
The IBIS (Infant Brain Imaging Study) Network is an NIH funded Autism Center of Excellence (HDO55741) and consists of a consortium of 8 Universities in the US and Canada. This work was supported by an NIH Autism Center of Excellence grant (NIMH and NICHD HD055741 to J.Pi.), Autism Speaks (6020) and the Simons Foundation (140209). Further support was provided by the National Alliance for Medical Image Computing (NA-MIC), funded by the NIH through grant U54 EB005149, the IDDRC Imaging and Participant Registry cores (NICHD HD003110 to J.Pi.) and R01 MH093510 (to J.R.P.Jr). We thank M. Burchinal and K. Y. Truong for their consultation on the statistical methods and approach. Given the large commitment of time and effort required by this study, we extend our appreciation to the families who have participated in this study and the numerous research assistants and staff who have contributed to this work.
Author information
Authors and Affiliations
Consortia
Contributions
All co-authors discussed the results, made critical contributions to the work and contributed to the writing of the manuscript. H.C.H., K.N.B., S.R.D., A.M.E., R.C.M., S.P., J.Pi., R.T.S., J. Pa. and D.W.S. contributed to the data collection. A.C.E., P.K. provided support for data management. B.C.M., S.H.K., M.S., D.L.C., A.C.E., V.S.F. and G.G. conducted image processing. H.G., B.C.M., S.H.K., M.S. and H.Z. analysed the data. H.C.H. wrote the manuscript with J.Pi., H.G., B.C.M., M.S. and with J.J.W., J.T.E., M.R.S., J.N.C., J.R.P.Jr, A.M.E., R.T.S. and L.Z. providing additional feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks M. Johnson, G. Ramsay, T. Yarkoni and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Visualization of cortical regions with surface area measurements among the top 40 features contributing to the linear sparse learning classification.
The cortical features produced by the deep learning approach (Fig. 3) are highly consistent with those observed using an alternative approach (linear sparse learning) shown here. Results from this alternative approach are included for comparison in Supplementary Tables 2 and 3.
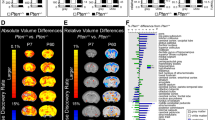
Extended Data Figure 2 Trajectories of TBV for males (left) and females (right).
For illustrative purposes, we provide plots for TBV for males and females from the same sample. The longitudinal trajectories of total brain volume (TBV) from 6 to 24 months for the three groups examined are shown with males and females displayed separately. The trajectory of TBV for males among the three groups is similar to the pattern we see in the full sample (Fig. 1). The female HR-ASD group is quite small (n = 2), which makes the pattern of trajectory difficult to interpret. These figures support the general similarity of the findings in the combined sample and the male-only sample. Red, HR-ASD; green, HR-neg; blue, LR. TBV is shown in mm3. The age (in months) is corrected by length (body size, in cm).
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Figures 1-10, Supplementary Tables 1-3 and Supplementary References. (PDF 1540 kb)
Rights and permissions
About this article
Cite this article
Hazlett, H., Gu, H., Munsell, B. et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 542, 348–351 (2017). https://doi.org/10.1038/nature21369
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21369
This article is cited by
-
Current and future applications of light-sheet imaging for identifying molecular and developmental processes in autism spectrum disorders
Molecular Psychiatry (2024)
-
Morphometric network-based abnormalities correlate with psychiatric comorbidities and gene expression in PCDH19-related developmental and epileptic encephalopathy
Translational Psychiatry (2024)
-
Differential cognitive and behavioral development from 6 to 24 months in autism and fragile X syndrome
Journal of Neurodevelopmental Disorders (2024)
-
The correlation between brain structure characteristics and emotion regulation ability in children at high risk of autism spectrum disorder
European Child & Adolescent Psychiatry (2024)
-
Cortical thickness abnormalities in autism spectrum disorder
European Child & Adolescent Psychiatry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.